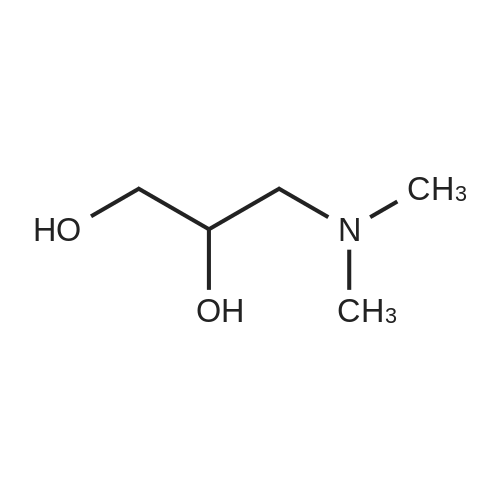
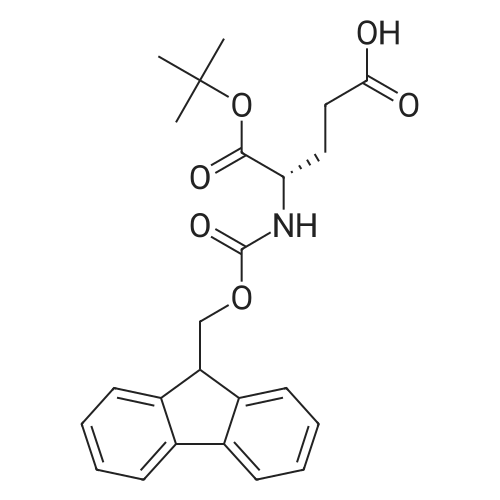
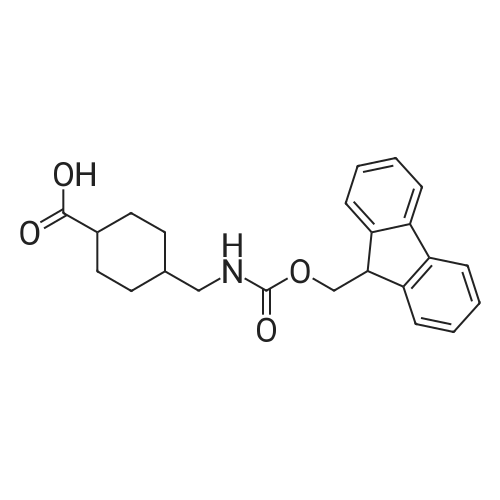
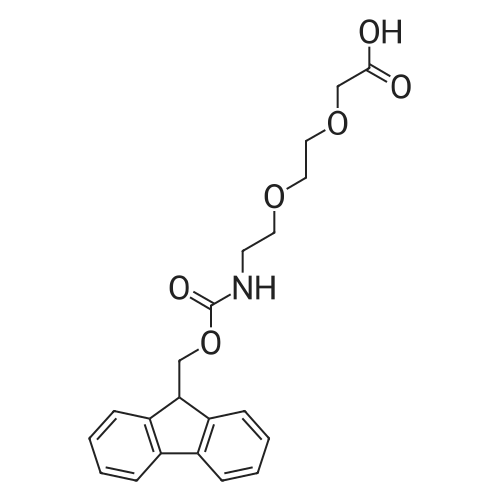
| 42% |
|
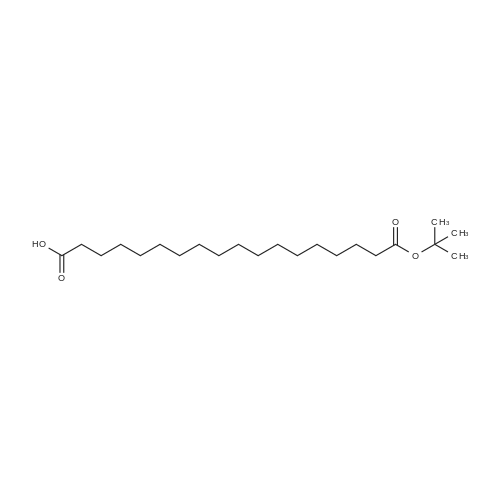
2-Chlorotrityl resin 100-200 mesh 1.8 mmol/g (1, 11.9 g, 21.4 mmol) was left to swell in dry dichloromethane (80 ml) for 20 minutes. A solution of {2-[2-(9H-fluoren-9ylmethoxycarbonylamino)-ethoxy]-ethoxy}-acetic acid (Fmoc-OEG-OH, 5.50 g, 14.3 mmol) and N,N-diisopropylethylamine (9.44 ml, 54.2 mmol) in dry dichloromethane (70 ml) was added to resin and the mixture was shaken for 4 hours. Resin was filtered and 10 treated with a solution of N,N-diisopropylethylamine (4.97 ml, 28.5 mmol) in methanol/dichloromethane mixture (4: 1, 2 x 5 min, 2 x 57 ml). Then resin was washed with N,N-dimethylformamide (2 x 80 ml), dichloromethane (2 x 80 ml) and N,Ndimethylformamide (3 x 80 ml). Fmoc group was removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 30 min, 2 x 80 ml). Resin was 15 washed with N,N-dimethylformamide (3 x 80 ml), 2-propanol (2 x 80 ml) and dichloromethane (100 ml, 2 x 80 ml). Solution of {2-[2-(9H-fluoren-9ylmethoxycarbonylamino)-ethoxy]-ethoxy}-acetic acid (Fmoc-OEG-OH, 11.0 g, 28.5 mmol), 0-( 6-chloro-benzotriazol-1-yi)-N,N,N', N'-tetramethyluronium tetrafluoroborate (TCTU, 10.1 g, 28.5 mmol) and N,N-diisopropylethylamine (9.93 ml, 57.0 mmol) in N,N20 dimethylformamide (80 ml) was added to resin and mixture was shaken for 2 hours. Resin was filtered and washed with N,N-dimethylformamide (2 x 80 ml), dichloromethane (2 x 80 ml) and N,N-N,N-dimethylformamide (3 x 80 ml). Fmoc group was removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 30 min, 2 x 80 ml). Resin was washed with N,N-dimethylformamide (3 x 80 ml), 225 propanol (2 x 80 ml) and dichloromethane (100 ml, 2 x 80 ml). Solution of (S)-2-(9Hfluoren-9-ylmethoxycarbonylamino)-pentanedioic acid 1-tert-butyl ester (Fmoc-LGiu OtBu, 9.11 g, 21.4 mmol), 0-(6-chloro-benzotriazol-1-yi)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TCTU, 7.60 g, 21.4 mmol) and N,N-diisopropylethylamine (6.71 ml, 38.5 mmol) in N,N-dimethylformamide (80 ml) was added to resin and mixture was shaken for 1 hour. Resin was filtered and washed with N,N-dimethylformamide (2 x 80 5 ml), dichloromethane (2 x 80 ml) and N,N-dimethylformamide (2 x 80 ml). Fmoc group was removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 30 min, 2 x 80 ml). Resin was washed with N,N-dimethylformamide (3 x 80 ml), 2propanol (2 x 80 ml) and dichloromethane (100 ml, 2 x 80 ml). Solution of 4-[(9Hfluoren-9-ylmethoxycarbonylamino)methyl]cyclohexanecarboxylic acid (<strong>[188715-40-4]Fmoc-Trx-OH</strong>, 10 9.11 g, 21.4 mmol), 0-(6-chloro-benzotriazol-1-yi)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TCTU, 7.60 g, 21.4 mmol) and N,N-diisopropylethylamine (6.71 ml, 38.5 mmol) in N,N-dimethylformamide (80 ml) was added to resin and mixture was shaken for 1 hour. Resin was filtered and washed with N,N-dimethylformamide (2 x 80 ml), dichloromethane (2 x 80 ml) and N,N-dimethylformamide (2 x 80 ml). Fmoc group was removed by treatment with 20% piperidine in N,N-dimethylformamide (1 x 5 min, 1 x 30 min, 2 x 80 ml). Resin was washed with N,N-dimethylformamide (3 x 80 ml), 2propanol (2 x 80 ml) and dichloromethane (100 ml, 2 x 80 ml). Solution of dodecanedioic acid mono-tert-butyl ester (C12(0tBu)-OH, 6.13 g, 21.4 mmol), 0-(6chloro-benzotriazol-1-yi)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TCTU, 7.61 g, 20 21.4 mmol) and N,N-diisopropylethylamine (6.71 ml, 38.5 mmol) in dichloromethane/N,N-dimethylformamide mixture (4: 1, 80 ml) was added to resin and mixture was shaken for 1.5 hour. Resin was filtered and washed with N,Ndimethylformamide (6 x 80 ml), dichloromethane (4 x 80 ml), methanol (4 x 80 ml) and dichloromethane (7 x 80 ml). The product was cleaved from resin by treatment with 25 2,2,2-trifluoroethanol (80 ml) for 18 hours. Resin was filtered off and washed with dichloromethane (4 x 80 ml), dichloromethane/2-propanol mixture (1:1, 4 x 80 ml), 2propanol (2 x 80 ml) and dichloromethane (6 x 80 ml). Solutions were combined; solvent evaporated and crude product was purified by column chromatography (Silicagel 60, 0.040-0-063 mm; eluent: dichloromethane/methanol 1:0-9:1). The pure product (2) 30 was dried in vacuo and obtained as oil. Yield: 5.40 g (42%). RF (Si02, dichloromethane/methanol 9: 1): 0.30. 1H NMR spectrum (300 MHz, CDC13, dH): 7.45-7.31 (m, 1 H); 7.10-6.97 (m, 1 H); 6.716.60 (m, 1 H); 5.70-5.58 (m, 1 H); 4.43-4.31 (m, 1 H); 4.15 (s, 2 H); 4.01 (s, 2 H); 35 3.79-3.31 (m, 16 H); 3.13-3.08 (m, 2 H); 2.28-1.79 (m, 11 H); 1.71-1.51 (m, 4 H); 1.46 (s, 9 H); 1.44 (s, 9 H); 1.25 (bs, 12 H); 1.05-0.88 (m, 2 H).LC-MS purity: 100%. LC-MS Rt (Sunfire 4.6 mm x 100 mm, acetonitrile/water 50:50 to 100:0 + 0.1% FA): 2.16 min. LC-MS m/z: 903.0 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping