| 50% |
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃; |
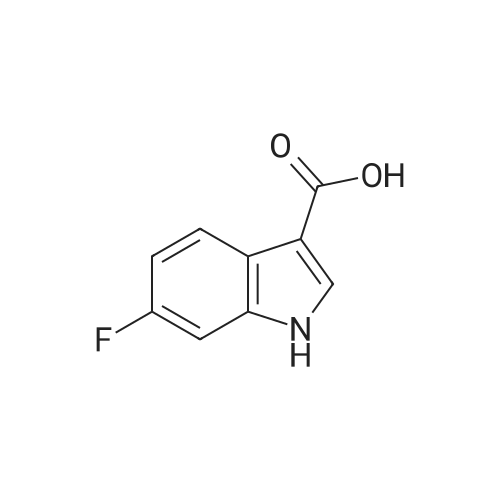
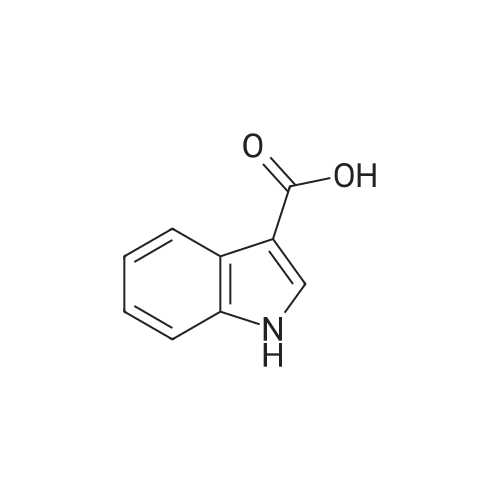
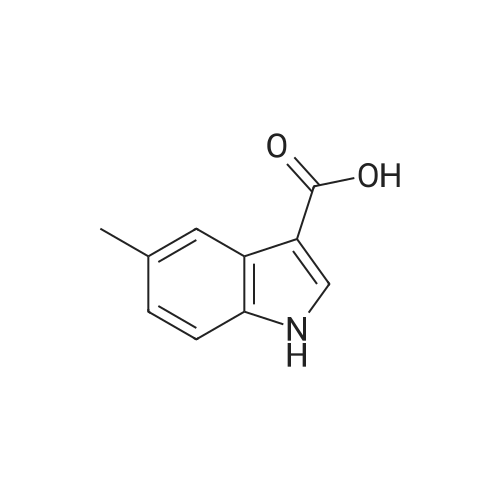
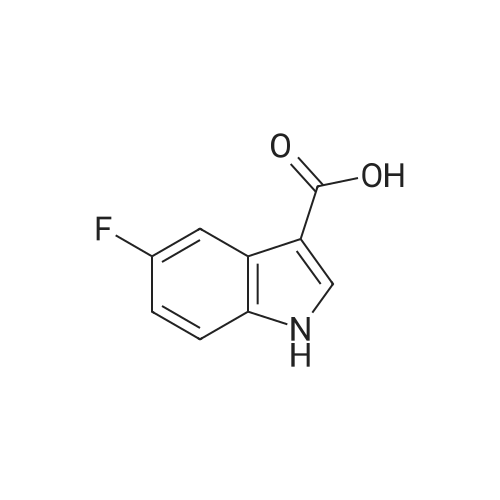
To a stirred solution of indolic amine (gb) (72 mg, 0.3 mmol) and <strong>[23077-43-2]5-fluoro-1H-indole-3-carboxylic acid</strong> (2f) (59 mg, 0.33 mmol) in 5 mL of dry CH2Cl2, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI, 5 mg, 0.33 mmol) was added at room temperature. The resulting mixture was stirred overnight. Then CH2Cl2 was evaporated then EtOAc (20 mL) and a 1M aqueous solution of hydrochloric acid (20 mL) were added. After extraction, organic phase was washed with an aqueous solution of hydrochloric acid, a 5% aqueous solution of Na2CO3, brine, dried over anhydrous MgSO4, filtered, and concentrated under vacuum. Purification of the crude product by column chromatography using EtOAc-pentane (from 1/9 to 8/2) yielded the pure bis-indole 5 (60 mg, 0.226 mmol) as a beige foam. Yield: 50%. IR (neat): 3455, 3240, 2975, 2870, 1740, 1595, 1530, 1460, 1425, 1205, 1170, 1080, 1005, 930, 850, 760 cm-1. 1H NMR (300 MHz, CD3OD-DMSO-d6): delta = 1.60 (d, J= 6.8 Hz, 3H), 5.49 (q, J= 6.8 Hz, 1H), 6.99 (dt, J = 2.6 and 9.0 Hz, 1H), 7.09 (dd, J = 1.9 and 8.7 Hz, 1H), 7.19-7.23 (m, 2H), 7.44 (dd, J = 4.4 and 9.0 Hz, 1H), 7.65 (dd, J= 2.6 and 9.6 Hz, 1H), 7.74 (d, J = 7.7 Hz, 3H), 8.18 (s, 1H) ppm. 13C NMR (75.5 MHz, CD3OD-DMSO-d6): delta = 21.3, 4.3, 106.9 (d, J = 25.1 Hz), 111.9 (d, J = 26.6 Hz), 112.3, 112.8, 114.0 (d, J = 9.8 Hz), 121.2, 121.4, 121.5, 124.6, 126.2, 127.5, 134.7, 134.9, 135.1, 160.0 (d, J= 235.1 Hz), 168.8, 175.5 ppm. LRMS (ESI): m/z = 422 and 424 [(M+Na)+]. |
| 50% |
With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; In dichloromethane; at 20℃; |
To a stirred solution of indolic amine (gb) (72 mg, 0.3 mmol) and 5-fluoro-lH-indole-3- carboxylic acid (2f) (59 mg, 0.33 mmol) in 5 mL of dry CH2CI2, l-ethyl-3-(3- dimethylaminopropyl)carbodiimide (EDCI, 5 mg, 0.33 mmol) was added at room temperature. The resulting mixture was stirred overnight. Then CH2CI2 was evaporated then EtOAc (20 mL) and a 1M aqueous solution of hydrochloric acid (20 mL) were added. After extraction, organic phase was washed with an aqueous solution of hydrochloric acid, a 5% aqueous solution of Na2C03, brine, dried over anhydrous MgSC>4, filtered, and concentrated under vacuum. Purification of the crude product by column chromatography using EtOAc-pentane (from 1/9 to 8/2) yielded the pure bis-indole 5 (60 mg, 0.226 mmol) as a beige foam. Yield: 50%.IR (neat): 3455, 3240, 2975, 2870, 1740, 1595, 1530, 1460, 1425, 1205, 1170, 1080, 1005, 930, 850, 760 cm"1. 1H NMR (300 MHz, CD3OD-DMSO-d6): delta = 1.60 (d, J= 6.8 Hz, 3H), 5.49 (q, J = 6.8 Hz, 1H), 6.99 (dt, J= 2.6 and 9.0 Hz, 1H), 7.09 (dd, J = 1.9 and 8.7 Hz, 1H), 7.19-7.23 (m, 2H), 7.44 (dd, J= 4.4 and 9.0 Hz, 1H), 7.65 (dd, J= 2.6 and 9.6 Hz, 1H), 7.74 (d, J= 7.7 Hz, 3H), 8.18 (s, 1H) ppm. 13C NMR (75.5 MHz, CD3OD-DMSO-d6): delta = 21.3, 4.3, 106.9 (d, J = 25.1 Hz), 1 11.9 (d, J = 26.6 Hz), 112.3, 112.8, 114.0 (d, J = 9.8 Hz), 121.2, 121.4, 121.5, 124.6, 126.2, 127.5, 134.7, 134.9, 135.1, 160.0 (d, J = 235.1 Hz), 168.8, 175.5 ppm. LRMS (ESI): m/z = 422 and 424 [(M+Na)+]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping