| 95% |
With potassium carbonate; In ethanol; for 24.0h;Heating / reflux; |
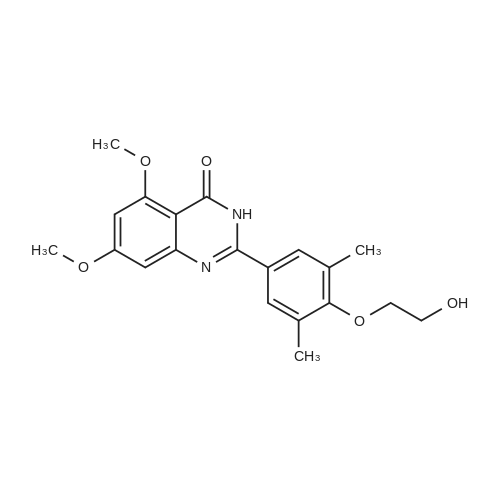
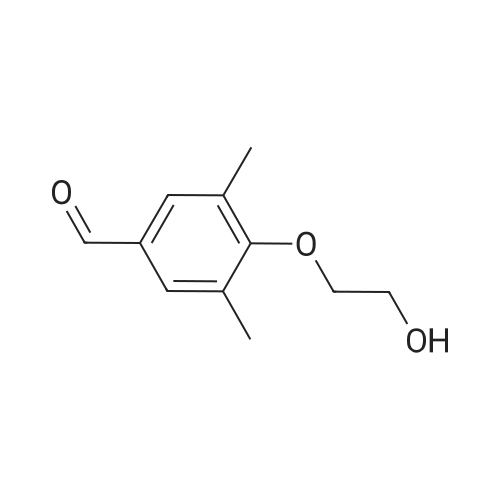
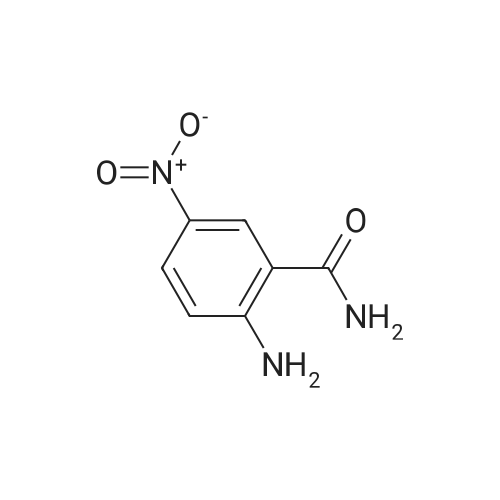
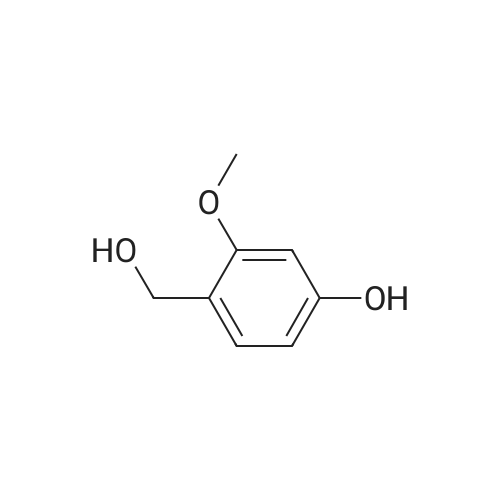
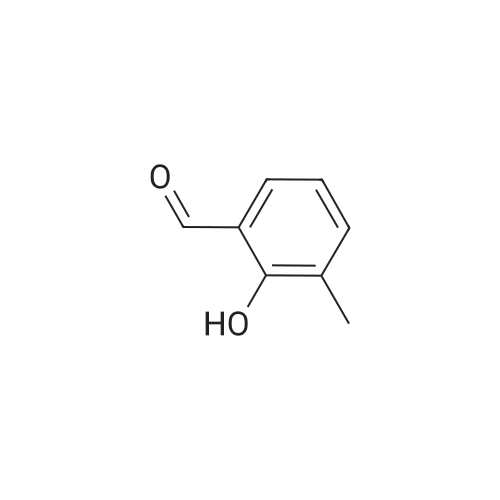
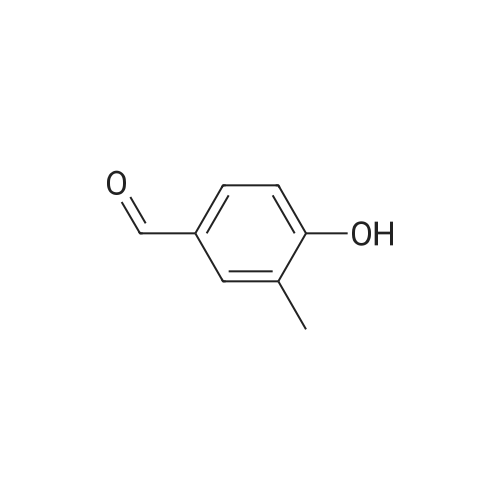
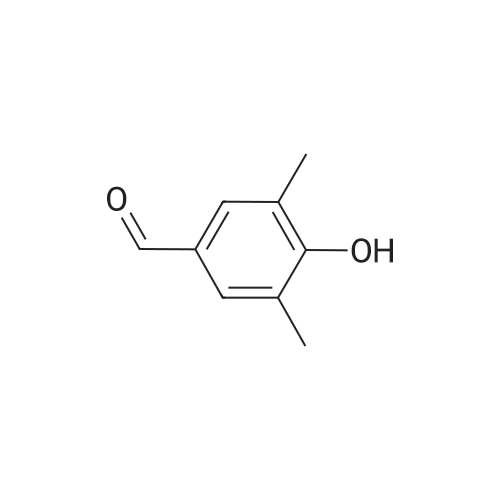
A solution of 2-amino-4,6-dimethoxybenzamide (0.60 g, 3.06 mmol) and 4-[2-(tert-butyidimethylsilanoxy)ethoxy]-3,5-dimethylbenzaldehyde (0.856 g, 2.78 mmol) in N,N-dimethyl formamide (20 mL) was stirred at 70 C. for 1 h. Iodine (0.846 g, 3.33 mmol) and potassium carbonate (0.384 g, 2.78 mmol) were added and the reaction mixture was stirred at 70 C. for 16 h. The reaction mixture was poured into ice, and extracted with ethyl acetate. The organic layer was washed with water, brine, and dried over anhydrous Na2SO4. Removal of the solvent gave the crude product which was purified by column chromatography to give 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethoxyquinazolin-4(3H)-one (444 mg, 39%) as a white solid. Selected data: 229-231 C.Alternatively, 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethoxyquinazolin-4(3H)-one can be synthesized by the following method. In a 2 L dry round-bottom flask with a reflux condenser and magnetic stirrer was placed 3,5-dimethyl-4-hydroxy benzaidehyde (26.9 g, 0.179 mol) in ethanol (350 mL). 2-chloroethanol (87.6 g, 1.074 mol) and K2CO3 (99 g, 0.716 mol) were added and the reaction mixture was heated to reflux for 24 h. The reaction mixture was cooled to room temperature and filtered. The solvent was removed under reduced pressure. The crude product was diluted with ethyl acetate and the organic layer was washed with water, brine, and dried over Na2SO4. Upon removal of solvent it gave 45 g of crude product. The crude product was purified by column chromatography (silica gel 230-400 mesh; 50% ethyl acetate in hexane as eluent) to give 33.3 g (95%) of product. To a solution of 2-amino-4,6-dimethoxy-benzamide (33.45 g, 0.170 mol) and 4-(2-hydroxy ethoxy)-3,5-dimethyl benzaldehyde (33.3 g, 0.170 mol) in N,N-dimethyl acetamide (300 mL), NaHSO3 (33.3 g, 0.187 mol) and p-TSA (3.2 g, 17.1 mmol) were added and the reaction mixture was heated at 150 C. for 14 h. The reaction was cooled to room temperature. The solvent was removed under reduced pressure. The residue was diluted with water and stirred for 30 min at room temperature. The solids separated were filtered and dried to give crude product. The crude product was purified by column chromatography (silica gel 230-400 mesh; 5% methanol in CH2Cl2 as eluent) to give 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethoxyquinazolin-4(3H)-one (33 g, 52%). |
| 95% |
With potassium carbonate; In ethanol; for 24.0h;Reflux; |
Alternatively, 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethoxyquinazolin-4(3H)-one can be synthesized by the following method. In a 2 L dry round-bottom flask with a reflux condenser and magnetic stirrer was placed 3,5-dimethyl-4-hydroxy benzaldehyde (26.9 g, 0.179 mol) in ethanol (350 mL). 2-chloroethanol (87.6 g, 1.074 mol) and K2CO3 (99 g, 0.716 mol) were added and the reaction mixture was heated to reflux for 24 h. The reaction mixture was cooled to room temperature and filtered. The solvent was removed under reduced pressure. The crude product was diluted with ethyl acetate and the organic layer was washed with water, brine, and dried over Na2SO4. Upon removal of solvent it gave 45 g of crude product. The crude product was purified by column chromatography (silica gel 230-400 mesh; 50% ethyl acetate in hexane as eluent) to give 33.3 g (95%) of product. To a solution of 2-amino-4,6-dimethoxy-benzamide (33.45 g, 0.170 mol) and 4-(2-hydroxy ethoxy)-3,5-dimethyl benzaldehyde (33.3 g, 0.170 mol) in N,N-dimethyl acetamide (300 mL), NaHSO3 (33.3 g, 0.187 mol) and p-TSA (3.2 g, 17.1 mmol) were added and the reaction mixture was heated at 150 C. for 14 h. The reaction was cooled to room temperature. The solvent was removed under reduced pressure. The residue was diluted with water and stirred for 30 min at room temperature. The solids separated were filtered and dried to give crude product. The crude product was purified by column chromatography (silica gel 230-400 mesh; 5% methanol in CH2Cl2 as eluent) to give 2-(4-(2-hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethoxyquinazolin-4(3H)-one (33 g, 52%). |
| 95% |
With potassium carbonate; In ethanol; for 24.0h; |
At 70deg.] C 2-Amino-4,6-dimethoxy-benzamide(0.60g, 3.06mmol) and 4- [2- (tert-butyldimethylsilyloxy)ethoxy]-3,5-dimethyl-benzaldehyde(0.856g,2.78mmol)in N, N- dimethylformamide(20 mL) was stirred for 1hour. Was added iodine (0.846g, 3.33mmol)and potassium carbonate (0.384g, 2.78mmol), thereaction mixture was at 70 stirred for 16 hours. The reactionmixture was poured onto ice, extracted with ethyl acetate. With water, theorganic layer was washed with brine, dried over anhydrous Na 2SO 4dry. The solvent was removed to give acrude product, which was purified by column chromatography to give a white solid of 2- (4- (2-hydroxyethoxy) -3,5-dimethylphenyl) -5,7-methoxy-quinazolin -4 (3H) - one (444mg, 39%). Selected data: 229-231 . Alternatively, 2- (4-(2-hydroxyethoxy)-3,5-dimethyl-phenyl)-5,7-dimethoxy-quinazolin -4 (3H) - onecan be synthesized by the following method. In ethanol (350 mL of) of 3,5-dimethyl-4-hydroxy-benzaldehyde(26.9g, 0.179mol) disposed dried 2L round bottomed flask with refluxcondenser and magnetic stirrer. 2-chloro-ethanol(87.6g, 1.074mol) and K 2CO 3(99g, 0.716mol),the reaction mixture was heated at reflux for 24 hours. The reaction mixture was cooled toroom temperature and filtered. The solvent was removed under reduced pressure.The crude product was diluted with ethyl acetate, and the organic layer waswashed with water, brine, Na 2SO 4dry. 45g crude product obtained after removalof the solvent. The crude product was purified by column chromatography (silicagel 230-400 mesh; with 50% hexanes in ethyl acetate as eluent) to afford 33.3g (95%) of product. 2-amino-4,6-dimethoxy - benzamide (33.45g, 0.170mol)and 4- (2-hydroxyethoxy) -3,5-dimethyl benzaldehyde(33.3g, 0.170 mol) was added NaHSO3(33.3g,0.187mol) in N,N- dimethylacetamide (300mL) solution of and p-TSA(3.2g, 17.1mmol), at 150 reaction mixturewas heated for 14 hours.The reaction was cooled to room temperature. The solvent was removed underreduced pressure. The residue was diluted with water, followed by stirring at room temperature for30 minutes. The solid was isolated by filtration and dried to give the crudeproduct. The crude product was purified by column chromatography (silica gel230-400 mesh; used in CH 2Cl 25% methanol as eluent) to afford 2- (4- (2-hydroxyethoxy) -3,5-dimethyl-phenyl)-5,7-dimethoxy-quinazoline -4 (3H) - one (33g, 52%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping