貨號:P001107
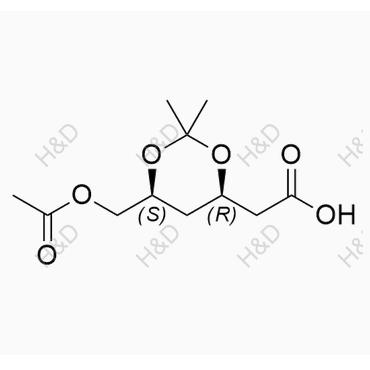
中文名稱:匹伐他汀雜質(zhì)107
英文名稱:Pitavastatin Impurity 107
CAS號:402508-35-4
純度:>95%
用途:供新藥研究及實驗使用
隨貨:提供COA�����,質(zhì)譜����,氫譜�����,HPLC。
恒豐萬達���,中國雜質(zhì)對照品引領者�����!
恒豐萬達作為一家擁有自主藥物雜質(zhì)對照品品牌的公司����,擁有雜質(zhì)對照品自主研發(fā)能力�。公司擁有多種重點雜質(zhì)現(xiàn)貨,價格優(yōu)�����,質(zhì)量保證�����。
如果您有雜質(zhì)現(xiàn)貨詢價�����,雜質(zhì)采購需求��,歡迎聯(lián)系:18367470722(劉經(jīng)理)
匹伐他汀雜質(zhì)正是恒豐萬達優(yōu)勢項目之一��,以上是匹伐他汀雜質(zhì)詳細信息�,
匹伐他汀化合物為白色粉末,化學式為((3R�,5S,6E)-7-[2-環(huán)丙基-4(p- 氟苯基)-3-喹啉基]-3�,5-二羥基-6-庚酸)。
匹伐他汀(通常作為鈣鹽)是他汀的降血脂降低藥物類別的成員�����,在美國以商品名Livalo銷售�。 與其他他汀類似,它是HMG-CoA還原酶的抑制劑�����,HMG-CoA還原酶催化膽固醇合成的第一步���。
匹伐他汀對羥甲戊二酰輔酶A(HMG-CoA)還原酶有強力拮抗抑制作用���,能夠高效抑制人肝細胞HepG2中生成膽固醇的過程,從而阻礙膽固醇的合成����。匹伐他汀能在超低濃度下誘導低密度脂蛋白(LDL)受體mRNA的合成�����,使其數(shù)量增加���,導致LDL受體密度增大,從而促進LDL的清除�,使血漿LDL-C濃度及血漿總甘油三酯濃度降低。
如果您有雜質(zhì)現(xiàn)貨詢價����,雜質(zhì)采購需求,歡迎聯(lián)系:18367470722(劉經(jīng)理)
公司常規(guī)配置COA�,HPLC, H-NMR����,MS滿足基本需求。企業(yè)根據(jù)自身需求�����,可另外檢測C-NMR、TGA�、QNMR、IR�����、UV���、二維譜等。適用于藥物的分析與質(zhì)量控制�。
更多優(yōu)勢項目:羅庫溴銨,卡比多巴�,西替利嗪,環(huán)丙沙星��,克拉霉素���,克林霉素�����,達非那新���,地塞米松,依那普利,依普利酮��,恩氟沙星�,法莫替丁,氟替卡松���,格列美脲�,格列吡嗪����,格列齊特,加替沙星���,氫氯噻嗪����,鹽酸羥嗪,伊潘立酮�����,依托必利����,酮咯酸,蘭索拉唑,左氧氟沙星�����,氯雷他定�,利多卡因,拉西地平����,拉米夫定���,米氮平����,霉酚酸���,孟魯司特����,甲氨蝶呤�����,咪康唑,莫西沙星�,美洛昔康,美羅培南����,諾氟沙星,尼美舒利���,尼羅替比��,奧美拉唑�����,昂丹司瓊����,奧司他韋����,奧洛他定,氧氟沙星����,泮托拉唑��,帕羅西汀��,己酮可可堿�����,帕洛諾司瓊�,帕潘立酮���,培美曲塞����,泊沙康唑非等��。
現(xiàn)貨供應����,品質(zhì)優(yōu)����,圖譜全,歡迎咨詢����!
如果您有雜質(zhì)現(xiàn)貨詢價��,雜質(zhì)采購需求��,歡迎聯(lián)系:18367470722(劉經(jīng)理)
關鍵字: 恒豐萬達;匹伐他汀雜質(zhì)107;雜質(zhì)對照品;雜質(zhì)現(xiàn)貨;402508-35-4;
恒豐萬達醫(yī)藥科技有限公司致力于為醫(yī)藥科學研究領域提供產(chǎn)品和服務�。產(chǎn)品主要包括醫(yī)藥行業(yè)標準品���,雜質(zhì)對照品��,植物單體及對照品�����,進口試劑及耗材等��。公司與眾多 的試劑公司���、藥企、研發(fā)機構(gòu)及高校等建立了良好的合作關系�����,為客戶提供咨詢�,定制�,代理進出口等服務��。我們通過快速的交貨���,合理的價格,完善的技術支持和售后服務��,與國內(nèi)外眾多客戶建立了穩(wěn)定的業(yè)務往來����。