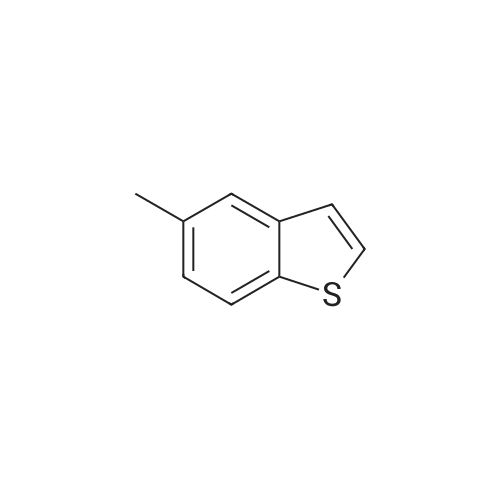
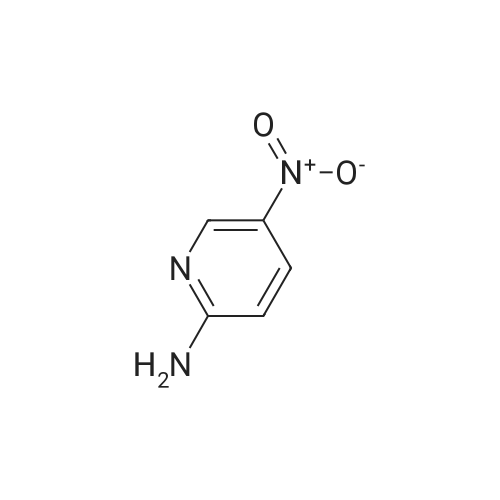
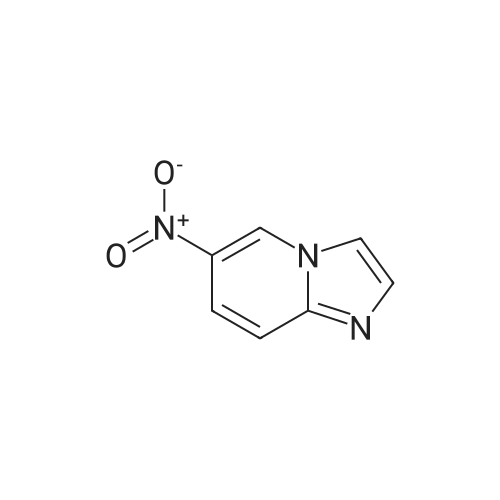
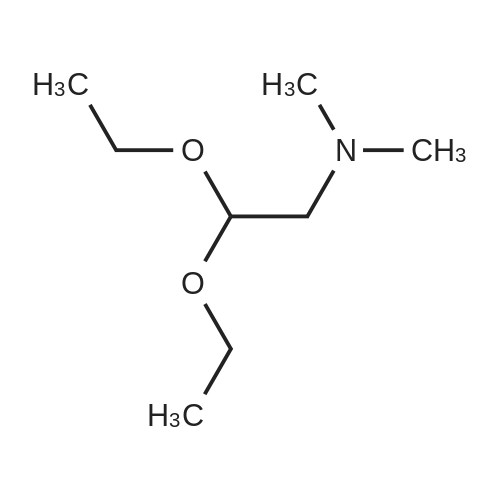
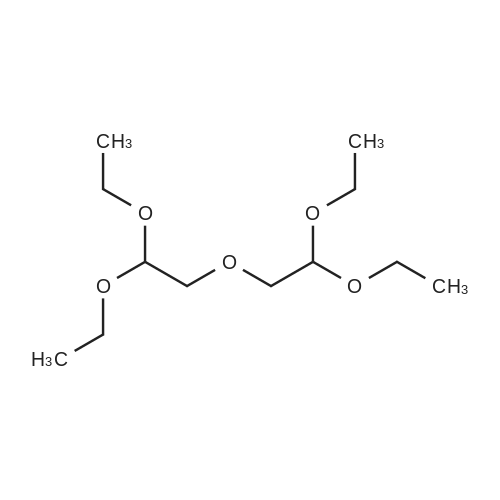
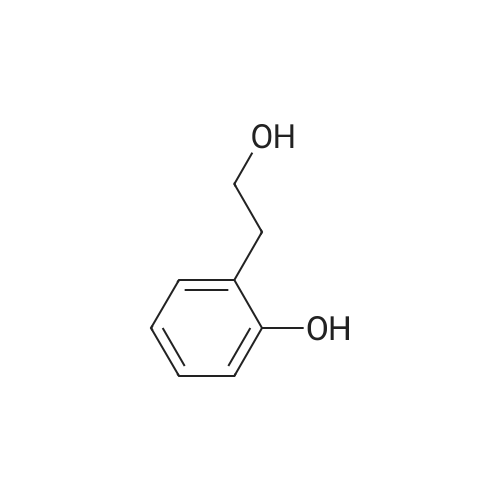
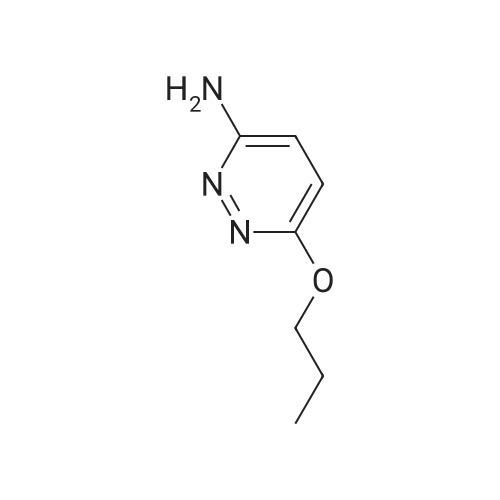
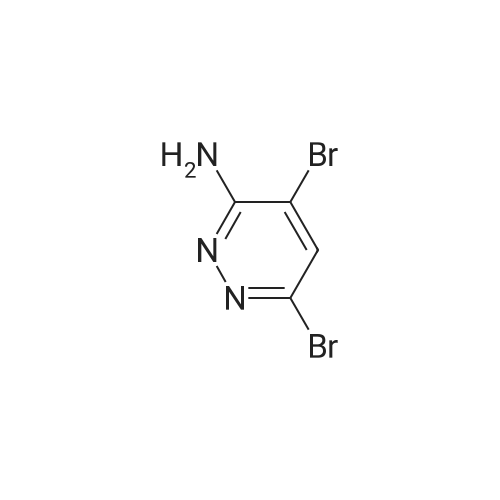
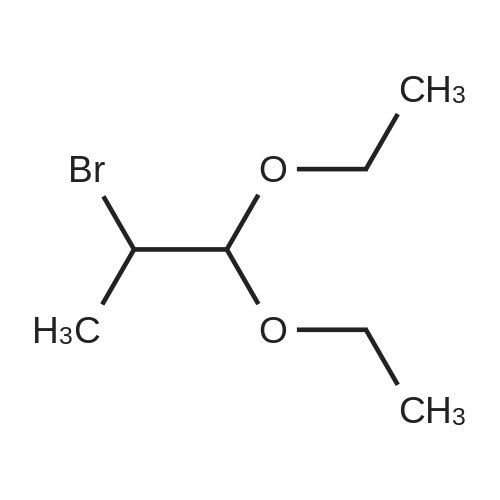
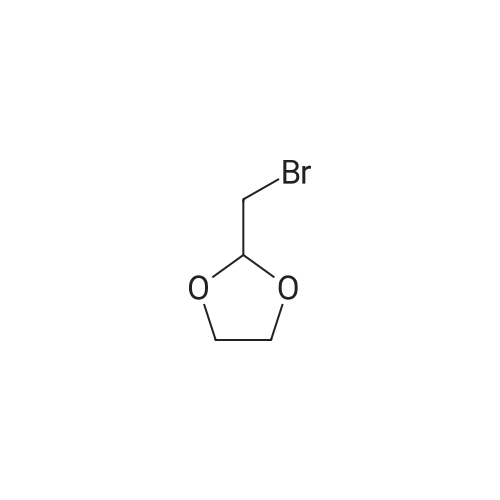
| 70% |
With sodium methylate; In N,N-dimethyl-formamide; at 90℃; for 4h; |
The synthesis of target compound 3 (Scheme 1C), started with the synthesis of a reported method for compound i.?3 2-Bromo-i,i-diethoxyethane (compound 10) was reacted with ethyl2-cyanoacetate to obtain compound 1 lwhich was cyclized to compound 12 using acetamidine hydrochloride under basic conditions. Chlorination of compound 12 using POC13 provided compound 13 in 80% yield. Displacement of the chloride of compound 13 with 4-methoxy-N- methyl aniline (compound 14) and catalytic amounts of HC1 in isopropanol, provided compound1. Methylation of compound 1 with Mel under basic conditions afforded compound 3 in 85% yield. The synthesis of target compound 5 (Scheme 1C), involved N-formylation of 4-methoxy- 2-methylanline (compound 15) to afford compound 16 in 70% yield. LAH reduction of compound 16 provided substituted aniline compound 17. Displacement of the chloride of compound 13 with anilines (compounds 15 and 17) and catalytic amounts of HC1 in isopropanol provided compounds 4 and 5 (75% and 70% respectively). |
| 70% |
With potassium carbonate; In N,N-dimethyl-formamide; at 150℃; |
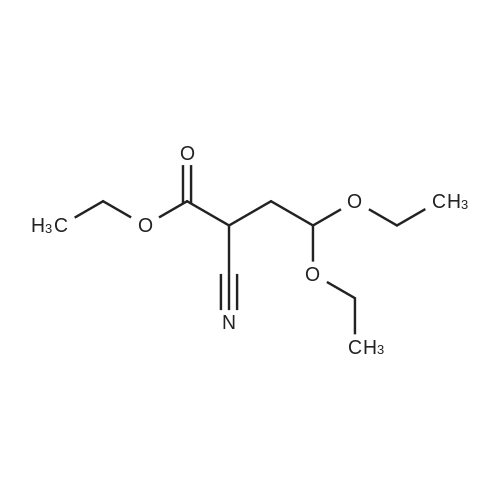
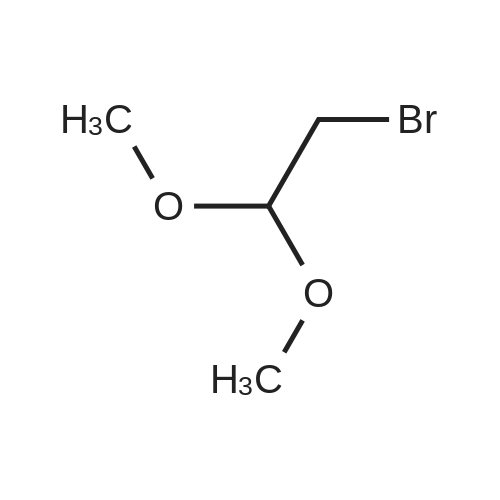
The 113 g (1mol)Ethyl cyanoacetate and 98.5 g (0.5 mol)Bromo acetal is dissolved in 450 g of DMF,Add 138 grams (1 mol) of potassium carbonate,Warm up to about 150 degrees, after the reaction is over,Cool to about 20 degrees and add 450 grams of water.Extracted twice with 500 g of chloroform,Wash once with saturated brine and dry.Vacuum distillation,78.1 g of ethyl 2-cyano-4,4-diethanolbutanoate were obtained in a yield of 70%. |
| 63% |
With potassium carbonate; sodium iodide;Reflux; |
Example A1; Preparation of 4-chloro-5-iodopyrrolo[2,3-d]pyrimidine in Accordance with the Following Reaction Scheme; (a) 130 ml (860 mmol) of bromoacetaldehyde diethyl acetal are refluxed for 10 h with ethyl cyanoacetate (430 ml, 4.04 mol), sodium iodide (8.1 g; 54.04 mmol) and potassium carbonate (115.9 g; 839 mmol). After cooling to room temperature (RT), the batch is stirred with 800 ml of water, the aqueous phase is extracted with diethyl ether, the combined organic phases are dried and evaporated. Chromatography gives 124.99 g (63%) of a colourless liquid ethyl 2-cyano-4,4-diethoxybutyrate. |
| 60% |
|
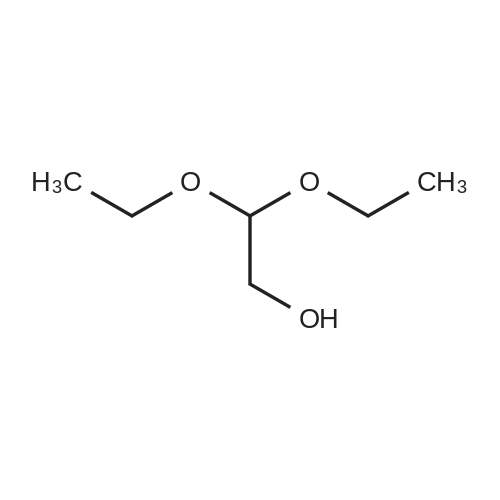
To a suspension of NaH (60% dispersion in mineral oil, 1.62 g, 40.5 mmol) in DMF (35 mL) and benzene (12 mL) was added ethyl cyanoacetate (4.7 mL, 44.2 mmol) dropwise at -10 0C. After stirring for 1 hour at room temperature, 2-bromo-1 ,1-diethoxyethane (5.6 mL, 0.82 equiv.) was added and the reaction mixture was heated at 100 0C for 2 hours. The reaction mixture was then cooled to room temperature and filtered. The filtrate was condensed, and water was added. The mixture was extracted with ether. The extracts were washed with brine, dried over MgSO4 and concentrated in vacuo. The crude material was purified by flash chromatography on silica gel (20% EtOAc/hexanes). The desired product was obtained as colorless oil (5 g, 60%). MS: (M + Na)/z = 252. |
| 60% |
|
Take a 500 mL two-necked flask and ethyl cyanoacetate (50 g, 0.44 mol) in anhydrous DMF(N, N-dimethylformamide) (300 mL) was added sodium ethoxide (36 g, 0.528 mol, 1.2 eq.) And sodium iodide (catalytic amount) at room temperature Stirring for 30min,2-Bromo-1,1-diethoxyethane was added(66.5 mL, 0.44 mol, 1 equiv)The temperature was raised to 90 C and stirred for 4 h. Cooled to room temperature, evaporated under reduced pressure to remove most of the DMF, add EA (ethyl acetate), stirring 10min, filter, take the filtrate to add saturation Salt water extraction. The organic phase was collected, dried, dried and dried (PE: EA = 12: 1, i.e., the volume ratio of petroleum ether: ethyl acetate was12) was obtained as a pale yellow oily product (61.3 g) in 60% yield. |
| 57% |
With potassium carbonate; sodium iodide; at 140 - 150℃; |
2-Cyano-4,4-diethoxy-butyric acid ethyl ester (4).; Bromoacetaldehyde diethylacetal (3, 541 g, 2.75 mol) was added to a suspension of powdered potassium carbonate (379.6 g, 2.75 mol, 1.0 equiv) and sodium iodide (33 g, 0.22 mol, 0.08 equiv) in ethyl cyanoacetate(2, 1.55 Kg, 13.75 mol, 5.0 equiv). Upon addition of the aldehyde to the reaction mixture, the resulting solution turned yellow. The reaction mixture was slowly heated to 140-150 C. collecting the volatile material in a Dean Stark trap. This material was discarded. Fairly vigorous gas evolution was observed to begin at 140 C. The reaction was monitored by G.C. and was observed to be near completion at 90 minutes. Heating was continued for an additional 45 minutes when gas evolution was observed to have ceased. The reaction mixture was then cooled to room temperature and partitioned between 4 L water and 2 L methyl tent-butyl ether (MTBE). The layers were separated and the aqueous layer was extracted with an additional 2 L of MTBE. The aqueous layer was checked for product by G.C. then discarded. The organic layers were dried over sodium sulfate, filtered and concentrated in vacuum. The crude product was purified by fractional distillation (91-105 C. (at) 0.53-0.65 mm/Hg) to afford 2-cyano-4,4-diethoxy-butyric acid ethyl ester (4, 359.4 g, 630.5 g theoretical, 57%) as a oil. For 4: 1H NMR (DMSO-d6, 300 MHz) delta ppm 4.60 (t, 1H, J=5.6 Hz), 4.15 (m, 3H), 3.59 (m, 2H), 3.45 (m,1H), 2.11 (t, 2H, J=6.2 Hz), 1.22 (t, 3H, J=6.9 Hz), 1.10 (dt, 6H, J=7.1, 6.9 Hz). |
| 51.0% |
With potassium carbonate; sodium iodide; for 24h;Reflux; |
Preparation of intermediate compound (87) a. A mixture of ethyl cyanoacetate 81 (227.97 g, 2015.52 mmol), bromo acetaldehyde diethyl ether (80) (80 g, 405.94 mmol), potassium carbonate (55.99 g, 405.13 mmol) and sodium iodide (4 g, 26.67 mmol) was refluxed for 20 h (CO2 evolution was observed during the reaction). The reaction mixture was stirred at reflux for additional 4 h after the evolution of CO2 has ceased. The reaction was cooled to room temperature, diluted with water (400 mL) and diethyl ether (400 mL). The organic layer was separated and the aqueous layer was extracted with diethyl ether (250 mL). The ether layers were combined washed with water (2 x 100 mL), brine (200 mL), dried, filtered and concentrated in vacuum. The product obtained was distilled under vacuum to furnish ethyl-2- cyano-4, 4-diethoxybutanoate (82) (47.5 g, 51.0 %) as a colorless oil; B.P: 103 C/1 mm Hg. 1HNMR (300 MHz, DMSO) delta 4.61 (t, J= 5.7, IH), 4.24 - 4.08 (m, 3H), 3.67 - 3.54 (m, 2H), 3.53 - 3.40 (m, 2H), 2.12 (t, J= 6.0, 2H), 1.23 (t, J= 7.1, 3H), 1.11 (td, J= 4.9, 7.0, 6H); IR (neat): 3482, 2980, 2901, 2361, 2252, 1749, 1446, 1374, 1262, 1218, 1128, 1062 and 857 cm"1; MS (ES"): 263.6 (M + 35); Analysis: CaIc for CnHi9NO4.0.25 H2O: C, 56.51; H, 8.40; N, 5.99; Found: C, 56.71; H, 8.16; N, 5.96. |
| 29.2% |
With potassium carbonate; potassium iodide; for 12h;Reflux; |
Step 5: Preparation of ethyl 2-cyano-4,4-diethoxybutanoate A mixture of ethyl 2-cyanoacetate (1000 g, 8.84 mol), 2-bromo-1,1-diethoxyethane (400 g, 2.03 mol), KI (33.4 g, 0.201 mol) and K2CO3 (280 g, 2.03 mol) was heated to reflux for 12 hrs. The reaction mixture was diluted with CH2Cl2 (1000 mL) and the resulting precipitate was filtered off and the filtrate was washed with brine and dried over anhydrous Na2SO4. The solvent was removed in vacuo and the residue distilled to give the title compound (136 g, 29.2% yield) as a light yellow oil that was used as is in the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +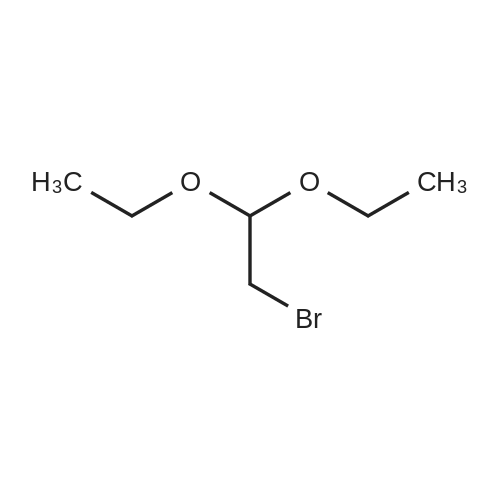

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping