| 12% |
|
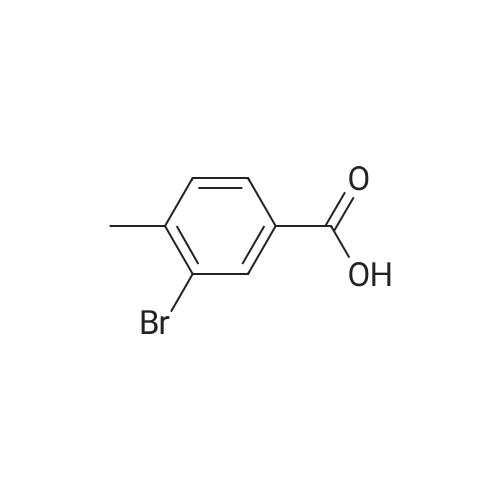
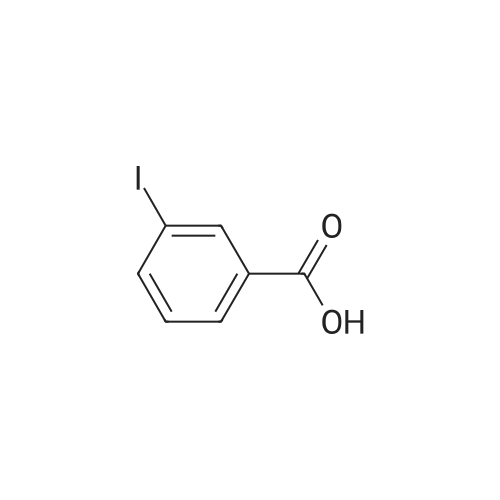
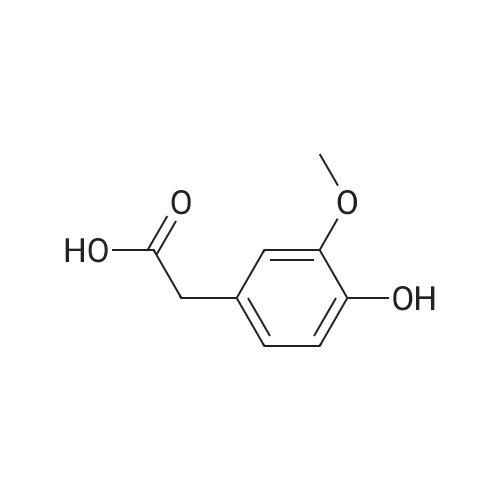
General procedure: General Procedure A for Rink Amide Resin Activation. Rink amide resin (Advanced ChemTech) was mixed with DCM (1 mL per 100 mg resin) and then shaken for 30 minutes. After activation, resin was washed three times with DMF (1 mL per 100 mg resin). [0079] General Procedure B for the Removal of the Fmoc Group from the Rink Amide Resin. Rink amide resin was mixed with 20% piperidine in DMF (1 mL per 100 mg resin) and shaken for 30 minutes, and then washed with DMF (1 mL per 100 mg resin, 3 times), isopropanol (1 mL per 100 mg resin, 3 times), and DCM (1 mL per 100 mg resin, 3 times) sequentially. The removal of the Fmoc group was confirmed by the ninhydrin test.General Procedure C for the Removal the Alloc Group from the Rink Amide Resin. The resin (200 mg) was washed with DCM (2 mL, 5 times) and shaken under 2 overnight with a solution of tetrakis(triphenylphosphine)palladium(0) (10 mg), AcOH (0.5 mL), and NMM (0.2 mL) in DCM (10 mL). The resin was then washed with DMF (2 mL, 3 times), isopropanol (2 mL, 3 times), and DCM (2 mL, 3 times). The removal of the Alloc group was confirmed by the ninhydrin test.General Procedure E for the Coupling of Carboxylic Acids to the Rink Amide Resin. Carboxylic acids (5 equiv, 0.5 M in DMF) were first mixed with HBTU (5 equiv, 0.5 M in DMF), HOBt (5 equiv, 0.5 M in DMF), and NMM (15 equiv, 1.5 M inDMF). The mixed solution was then added to the resin and shaken for 2 hours. The resin was then washed with DMF (1 mL per 100 mg resin, 3 times), isopropanol (1 mL per 100 mg resin, 3 times), and DCM (1 mL per 100 mg resin, 3 times). The completion of the coupling reaction was confirmed by the ninhydrin test. [0083] General Procedure F for Peptide Cleavage from the Rink Amide Resin. The resin was washed with DCM (1 mL per 100 mg resin, 5 times) and subsequently shaken with a solution of 95% TFA, 2.5% TIS, and 2.5% H20 (1 mL per 100 mg resin) for 2 hours. The resin was removed by filtration, and the TFA was evaporated under vacuum. The crude peptide was obtained after trituration with diethyl ether (5 mL per 100 mg resin, 2 times) Compound 8 was synthesized using standard Fmoc chemistry on the Rink amide resin (Figure 18). The resin (200 mg, 0.7 mmol/g loading) was first activated by DCM (General procedure A). The Fmoc group on the resin was removed by piperidine in DMF (General procedure B). The resin was then coupled with Fmoc-Dpr(Boc)-OH. The Fmoc group on the resin was removed by piperidine in DMF (General procedure B). The resin was then coupled with <strong>[147290-11-7]<strong>[147290-11-7]Fmoc-Orn(Alloc)</strong>-OH</strong>. The Fmoc group was removed (General procedure B) and Fmoc-Phe-OH was attached to resin (General procedure E). The Fmoc group was again removed (general procedure B) and the amine group on the F2Pmp residue was coupled with BMBA (general procedure E). The resin was treated with Pd(0) for the deprotection of Alloc group (general procedure C). 3- Iodobenzoic acid (mlBA) was attached to resin (general procedure E). The resin was treated with TFA (general procedure F) to give the crude peptide intermediate, which was treated with a mixture of HVA (0.5 M in DMF, 100 muGamma), HBTU (0.5 M in DMF, 100 muGamma), HOBt (0.5 M in DMF, 100 muGamma) and NMM (1.5 M in DMF, 100 muGamma) to give the crude product 8. The crude product was purified by HPLC to afford 8 (15.8 mg, 12% yield). The assignment of proton NMR utilized additional information from COSY. 1H NMR (500 MHz, DMSO-d6): S= 8.70 (d, J= 8.1 Hz, 1 H, BMBA-NH), 8.60-8.55 (m, 1 H, mlBA- NH), 8.35 (d, J= 6.9 Hz, 1 H, Phe-NH), 8.19 (s, 1 H, mlBA-ArH), 8.04-7.95 (m, 2 H, Orn-NH, BMBA-ArH), 7.91-7.83 (m, 3 H, mlBA-ArH, HVA-NH), 7.67 (d, J= 7.5 Hz, 1 H, BMBA-ArH), 7.40-7.15 (m, 9 H, BMBA-ArH, Phe-ArH, -CONH2 , mlBA-ArH), 6.77 (s, 1 H, HVA-ArH), 6.63 (d, J= 7.6 Hz, 1 H, HVA-ArH), 6.58 (d, J= 7.6 Hz, 1 H, HVA- ArH), 4.78-4.72 (m, 1 H, Phe-CaH), 4.30-4.22 (m, 2 H, Dpr-CaH, Orn-CaH), 3.70 (s, 3 H, HVA-OCH3), 3.40-3.35 (m, 1 H, Dpr-CpHH'), 3.35-3.18 (m, HVA-CH2-CO, Dpr-CpHH', Omicronpiiota-OmicrondeltaEta2, Phe-CpHH'), 3.04-2.96 (m, 1 H, Phe-CpHH'), 2.35 (s, 3 H, BMBA-Ar-CH3), I.81-1.74 (m, 1 H, Orn-CpHH'), 1.68-1.52 (m, 3 H, Orn-CpHH', Orn-CYH2). 13C MR (125 MHz, DMSO-d6 ): S= 171.79, 171.39, 171.25, 171.17, 164.70, 164.52, 147.13, 144.94, 140.65, 139.46, 138.30, 136.49, 135.51, 133.34, 130.75, 130.32, 129.00, 127.92, 126.62, 126.54, 126.10, 123.78, 121.26, 1 15.05, 1 13.13, 94.53, 55.37, 54.77, 53.01, 52.80, 41.74, 40.57, 36.68, 28.99, 25.46, 22.26. MS (ESI): calculated for [M], 954, found [M+H]+ 955. HPLC purity analysis: > 95% (UV, lambda = 254 nm). Figure 18 depicts the synthesis of Compound 8: (a) 30% piperidine/DMF; (b) Fmoc-Dpr(Boc)-OH/HBTU/HOBt/NMM; (c) <strong>[147290-11-7]Fmoc-Orn(Alloc)</strong>- OH/HBTU/HOBt/NMM; (d) Fmoc-Phe-OH/HBTU/HOBt/NMM; (e) 3-bromo-4- methylbenzoic acid/HBTU/HOBt/NMM; (f) Pd(0)/NMM/AcOH; (g) 3 -iodobenzoic acid/HBTU/HOBt/NMM; (h) 95% TFA/H2O/TIS; (i) homovanillic acid/HBTU/HOBt/NMM. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping