Alternatived Products of [ 19767-45-4 ]
Product Details of [ 19767-45-4 ]
| CAS No. : | 19767-45-4 |
MDL No. : | MFCD00007535 |
| Formula : |
C2H5NaO3S2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | XOGTZOOQQBDUSI-UHFFFAOYSA-M |
| M.W : |
164.18
|
Pubchem ID : | 23662354 |
| Synonyms : |
Mesnum;Mesna;Ziken. Abbreviation: MSA. Code names: D7093;Uromitexan;Mucolene;Mucofluid;Mitexan;Mistabronco;Mistabron;Mexan;Mesnil;Filesna;Mesnum. US brand name: Mesnex. Foreign brand names: Ausobronc;mercaptoethane sulfonate;UCB 3983;2-Mercaptoethanesulfonate;HS-CoM;Coenzyme M
|
Application In Synthesis of [ 19767-45-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 19767-45-4 ]
- 1
-
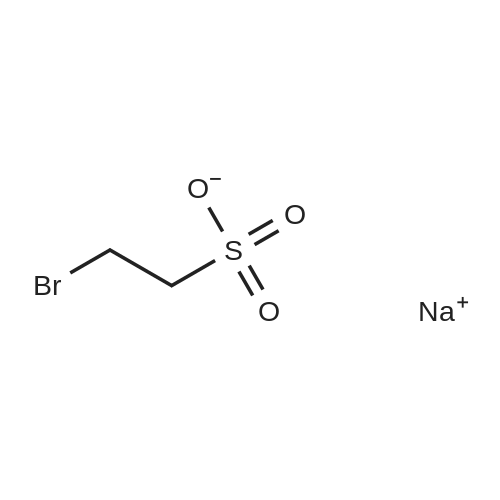 [ 4263-52-9 ]
[ 4263-52-9 ]

-
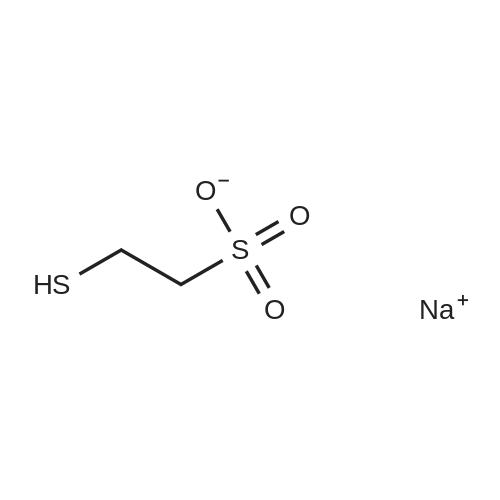 [ 19767-45-4 ]
[ 19767-45-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
EXAMPLE 2 Production of Disodium 2,2'-dithiobis Ethane Sulfonate To a mixture of 50.1 g of water with 24.4 g of thioacetic acid, 50.7 g of 25% aqueous sodium hydroxide solution were added dropwise at 10-30 C. This solution was added dropwise to a solution of 63.3 g of sodium 2-bromoethane sulfonate and 70 g of water at 50-70 C. and allowed to react at 80-90 C. for 2 hours. Thereto 54.2 g of 25% aqueous sodium hydroxide solution were added and allowed to react at refluxing temperature (about 105 C.) until the end of the reaction was confirmed by HPLC. After addition of 3.25 g of acetic acid, the reaction mixture was refluxed for 6 hours and then cooled to about 30 C. The pH of the mixture was adjusted to 7.3 with 25% sodium hydroxide solution. Oxygen was allowed to react with 260 mL of aqueous sodium 2-mercapto ethane sulfonate solution obtained above at about 30 C. and 0.5-0.6 MPa of oxygen pressure. When the end of the reaction was confirmed by HPLC, the reaction was stopped and the mixture was neutralized with acetic acid. The mixture was heated to about 70 C. and it was observed that the mixture had been dissolved. After that, the mixture was filtered with a filtering assistant agent (radiolite) and the filtering assistant agent was washed with 10 g of water. The mixture was concentrated under reduced pressure (about 10 kPa) at 70 C. When the amount of the distilled out water became 60 g, the concentration was stopped and it was observed that the mixture remained dissolved at about 75 C. Cooling the mixture, crystallization began at 60+-5 C. After aging for about 30 minutes, the mixture was cooled to 25 C. and the crystals were aged for 2 hours at 25 C. The crystals were filtered out and washed with 24 g of water being cooled to 2 C. and then 48 mL of 70% aqueous ethanol solution. Drying the crystals at about 70 C. afforded 39.1 g of substantially pure disodium 2,2'-dithiobis ethane sulfonate crystals. The yield was 77.6% after crystallization. The purity of the product was 99.4%. According to the present invention, compounds of Formula II, such as disodium 2,2'-dithiobis ethane sulfonate, can be produced by an efficient procedure from available, relatively less expensive raw compounds in good yield with high purity. The above details are not limitative of the invention, which is defined by the scope of the following claims. It will be appreciated by those skilled in the art that changes could be made to the embodiments described above without departing from the broad inventive concept thereof. It is understood, therefore, that this invention is not limited to the particular embodiments disclosed, but it is intended to cover modifications within the spirit and scope of the present invention as defined by the appended claims. |
- 2
-
 [ 555-30-6 ]
[ 555-30-6 ]

-
 [ 19767-45-4 ]
[ 19767-45-4 ]

-
C12H16NO7S2(1-)*Na(1+)
[ No CAS ]
- 3
-
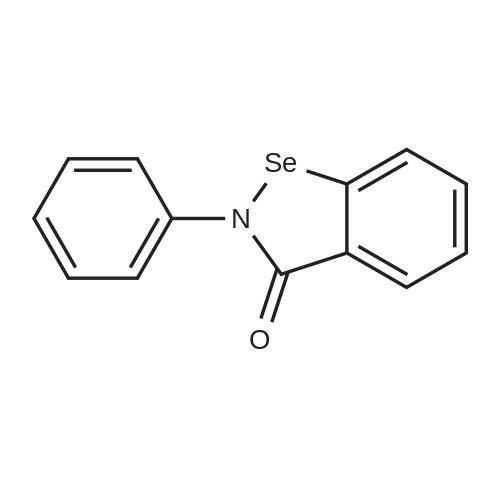 [ 60940-34-3 ]
[ 60940-34-3 ]

-
 [ 19767-45-4 ]
[ 19767-45-4 ]

-
C15H14NO4S2Se(1-)*Na(1+)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In methanol; dichloromethane; for 1h; |
Example 7The compound above was made by dissolving 2-phenyl- 1 ,2- benzisoselenazol-3(2H)-one (0.7156 g, 2.61 mmol) in 50 ml drydichloromethane, dissolving sodium 2-mercaptoethanesulfonate in 20 ml methanol, and mixing. After one hour the reaction was filtered through a medium glass frit and the filtrate evaporated to dryness. A portion of this (0.304 g) was dissolved in about 30 ml of methanol, then 0.3 ml of 4 M HCl in dioxane was added. The reaction was evaporated, then coevaporated three times with dichloromethane, then resuspended in 5 ml water and filtered through a medium glass frit. The precipitate was rinsed with an additional 5 ml of water and air- dried to yield 127 mg of a white solid characterized by HPLC-MS: m/z = 418.3. |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










