|
With potassium carbonate; In diethyl ether; hexane; water; pyrographite; ethyl acetate; N,N-dimethyl-formamide; |
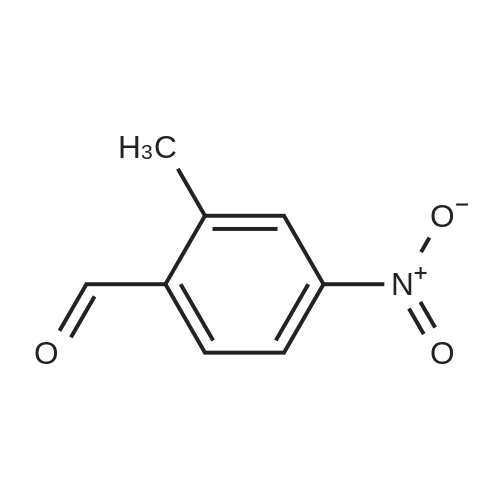
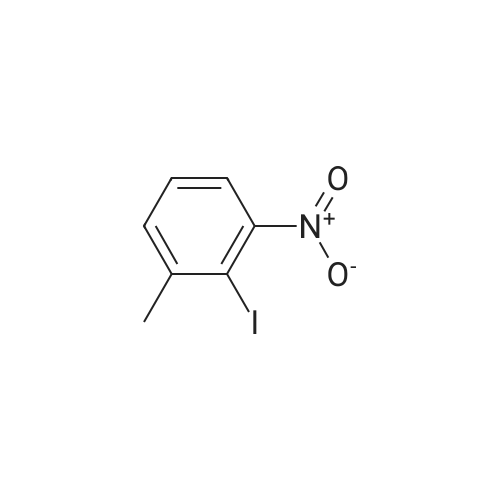
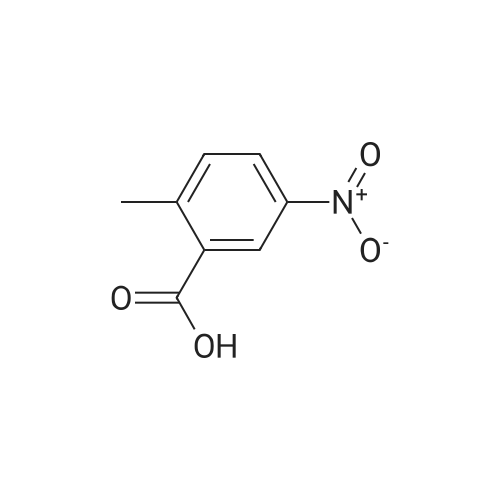
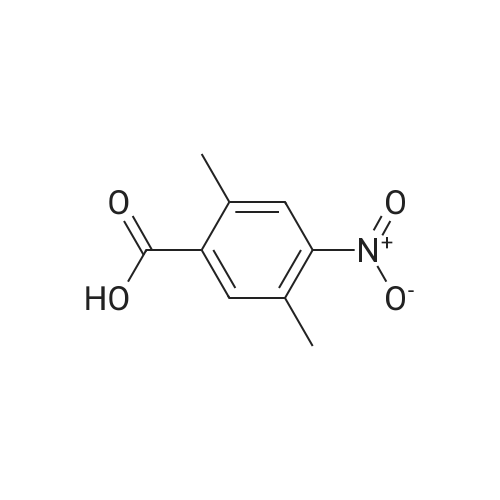
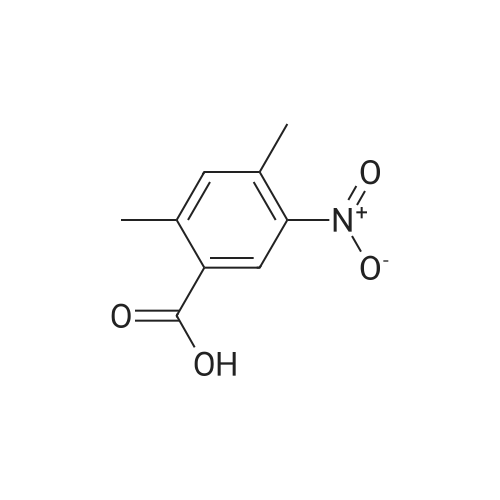
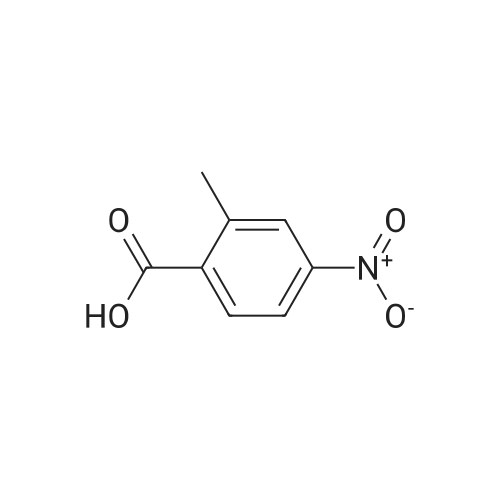
Part A. Preparation of 4-Nitro-2-methylbenzoic Acid. A mixture of 1.0 g (3.8 mmol) of 2-iodo-nitrotoluene, 2.1 g (15.2 mmol) potassium carbonate and 27 mg (0.038 mmol) of palladium(II) dichloride bis(triphenylphosphine) in a mixture of 5 mL of water and 10 mL of N,N-dimethylformamide. This was placed in a Fisher/Porter bottle under 15 psig of carbon monoxideand heated at 70 C. for 16 hours. The solution became homogeneous when heated. The reaction was cooled, diethyl ether and water was added, the organic layer separated and discarded. The aqueous layer was acidified with IN hydrohloric acid, extracted with ethyl acetate, washed with water, brine, dried over magnesium sulfate, filtered and concentrated to yield 0.5 g of crude material. This dissolved in ethyl acetate, hexane added and the resulting brown solid discarded. The filtrate was concentrated, and then recrystallized fom diethyl ether/hexane to afford 215 mg of 4-nitro-2-methylbenzoic acid, m/e=182 (M+H). |
|
With potassium carbonate; In diethyl ether; hexane; water; pyrographite; ethyl acetate; N,N-dimethyl-formamide; |
Part A. Preparation of 4-Nitro-2-methylbenzoic Acid A mixture of 1.0 g (3.8 mmol) of 2-iodo-nitrotoluene, 2.1 g (15.2 mmol) potassium carbonate and 27 mg (0.038 mmol) of palladium(II) dichloride bis(triphenylphosphine) in a mixture of 5 mL of water and 10 mL of N,N-dimethylformamide. This was placed in a Fisher/Porter bottle under 15 psig of carbon monoxideand heated at 70 C. for 16 hours. The solution became homogeneous when heated. The reaction was cooled, diethyl ether and water was added, the organic layer separated and discarded. The aqueous layer was acidified with 1N hydrohloric acid, extracted with ethyl acetate, washed with water, brine, dried over magnesium sulfate, filtered and concentrated to yield 0.5 g of crude material. This dissolved in ethyl acetate, hexane added and the resulting brown solid discarded. The filtrate was concentrated, and then recrystallized fom diethyl ether/hexane to afford 215 mg of 4-nitro-2-methylbenzoic acid, m/e=182(M+H). |
|
With potassium carbonate; In diethyl ether; hexane; water; pyrographite; ethyl acetate; N,N-dimethyl-formamide; |
Part A. Preparation of 4-Nitro-2-methylbenzoic Acid. A mixture of 1.0 g(3.8 mmol) of 2-iodo-nitrotoluene, 2.1 g(15.2 mmol) potassium carbonate and 27 mg(0.038 mmol) of palladium(II) dichloride bis(triphenylphosphine) in a mixture of 5 mL of water and 10 mL of N,N-dimethylformamide. This was placed in a Fisher/Porter bottle under 15 psig of carbon monoxideand heated at 70 C for 16 hours. The solution became homogeneous when heated. The reaction was cooled, diethyl ether and water was added, the organic layer separated and discarded. The aqueous layer was acidified with iN hydrohloric acid, extracted with ethyl acetate, washed with water, brine, dried over magnesium sulfate, filtered and concentrated to yield 0.5 g of crude material. This dissolved in ethyl acetate, hexane added and the resulting brown solid discarded. The filtrate was concentrated, and then recrystallized fom diethyl ether/hexane to afford 215 mg of 4-nitro-2-methylbenzoic acid, m/e=182(M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping