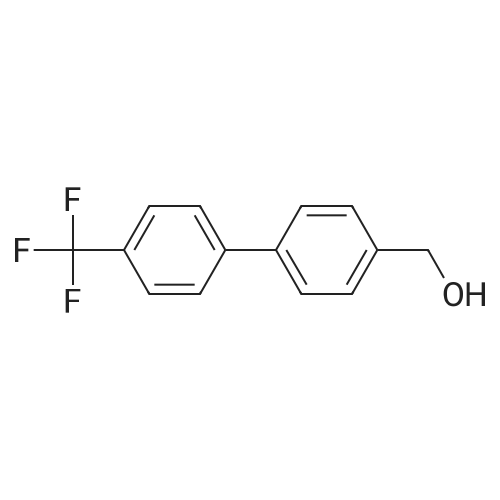
| 79% |
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃; for 4h;Inert atmosphere; |
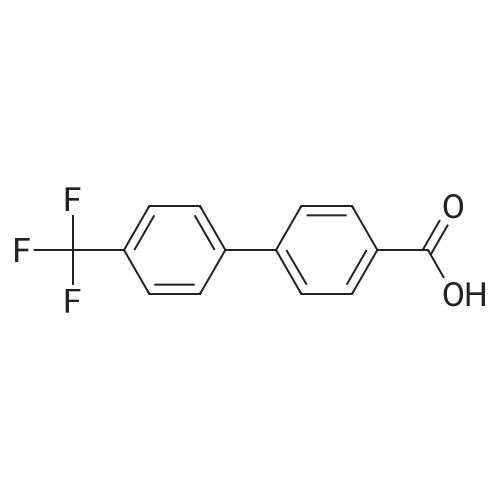
Under nitrogen atmosphere, at 0 C, to a stirring mixture of LiAlH4 (2.0 M THF solution, 3.0 mL, 6.00 mmol) in dry THF (10 mL), commercially available 4-[4- (trifluoromethyl)-phenyl]-benzoic acid (0.4 g, 1.5 mmol) in dry THF (10 mL) was added dropwise. The mixture was left to react at rt for 4 h, then at 0 C H20 (0.23 mL), 3.0 M KOH solution (0.23 mL) and H20 (0.77 mL) were very slowly added. The mixture was stirred for 1 h at 0 C, filtered to remove the solid residue, and the organic phase dried over Na2S04. The organic solution was again filtered, concentrated to dryness and the resulting crude product purified by column chromatography using a Teledyne ISCO apparatus, eluting with CyrEtOAc (from 100:0 to 70:30) to afford the title compound (0.3 g, 79%), as white solid. 1H NMR (DMSO-d6): delta 4.56 (d, J= 5.7 Hz, 2H), 5.25 (t, J= 5.7 Hz, 1H), 7.45 (d, J= 8.1 Hz, 2H), 7.70 (d, J = 8.1 Hz, 2H), 7.81 (d, J= 8.1 Hz, 2H), 7.89 (d, J= 8.1 Hz, 2H). |
| 79% |
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃; for 4h;Inert atmosphere; |
Step 1. Preparation of [4-[4-(trifluoromethyl)-phenyl]-phenyl]-methanol Under nitrogen atmosphere, at 0 C., to a stirring mixture of LiAlH4 (2.0 M THF solution, 3.0 mL, 6.00 mmol) in dry THF (10 mL), commercially available 4-[4-(trifluoromethyl)-phenyl]-benzoic acid (0.4 g, 1.5 mmol) in dry THF (10 mL) was added dropwise. The mixture was left to react at rt for 4 h, then at 0 C. H2O (0.23 mL), 3.0 M KOH solution (0.23 mL) and H2O (0.77 mL) were very slowly added. The mixture was stirred for 1 h at 0 C., filtered to remove the solid residue, and the organic phase dried over Na2SO4. The organic solution was again filtered, concentrated to dryness and the resulting crude product purified by column chromatography using a Teledyne ISCO apparatus, eluting with Cy:EtOAc (from 100:0 to 70:30) to afford the title compound (0.3 g, 79%), as white solid. 1H NMR (DMSO-d6): delta 4.56 (d, J=5.7 Hz, 2H), 5.25 (t, J=5.7 Hz, 1H), 7.45 (d, J=8.1 Hz, 2H), 7.70 (d, J=8.1 Hz, 2H), 7.81 (d, J=8.1 Hz, 2H), 7.89 (d, J=8.1 Hz, 2H). |
| 66.8% |
|
Description 8a: 4-(4-trifluoromethylphenyl)-benzyl alcohol (D8a); To a solution of 4-(4-trifluoromethylphenyl)-benzoic acid (Apollo, 3g, 11.27mmol) in THF (100ml) was added dropwise a solution of LiAIH4 1 M in THF (1 1.3ml, 11.27mmol) and the mixture was stirred at room temperature for 30 minutes. Water (50ml) was then added dropwise. The insoluble material was filtered on a Celite pad and washed with CH2CI2.The filtrate was washed with CH2CI2 and the organic phase was dried (Na2SO4) and concentrated under reduced pressure. The title compound was obtained as a white solid (1.9g, yield= 66.8%); 1H NMR (300MHz, DMSO d6, ppm): 7.9 (d, 2H), 7.85 (d, 2H), 7.7 (d,2H), 7.45 (d, 2H), 5.3 (t, 1 H), 4.55 (d, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping