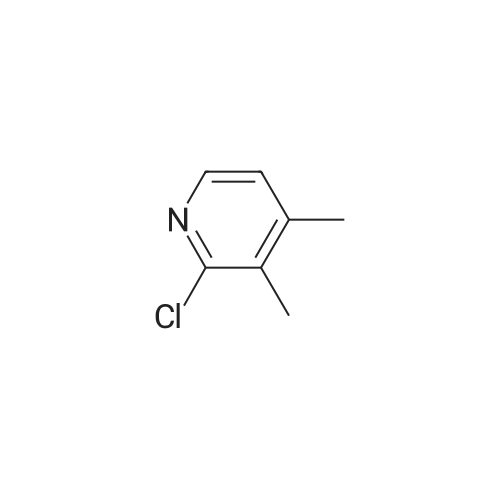
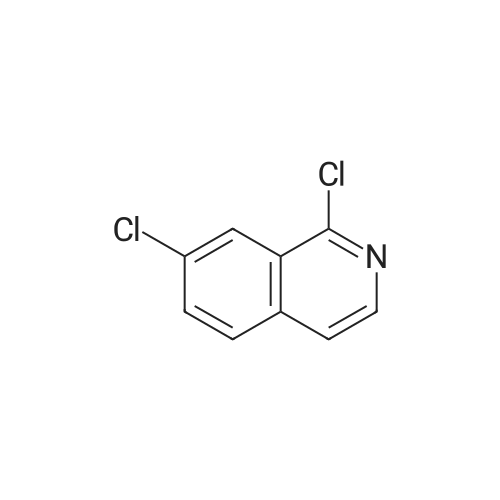
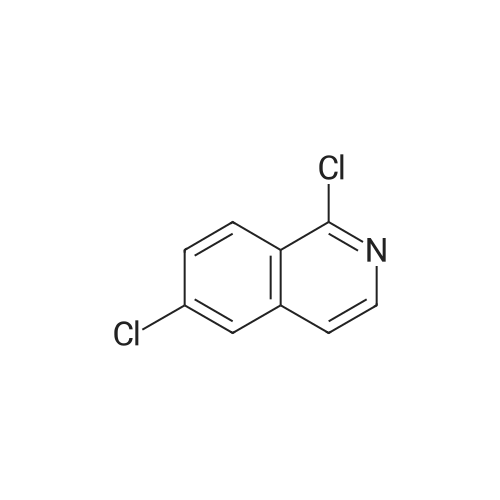
|
|
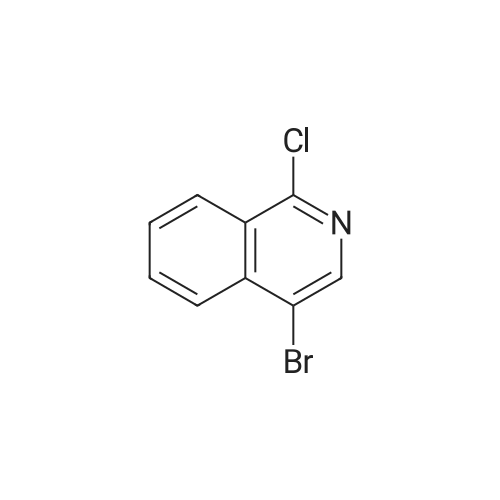
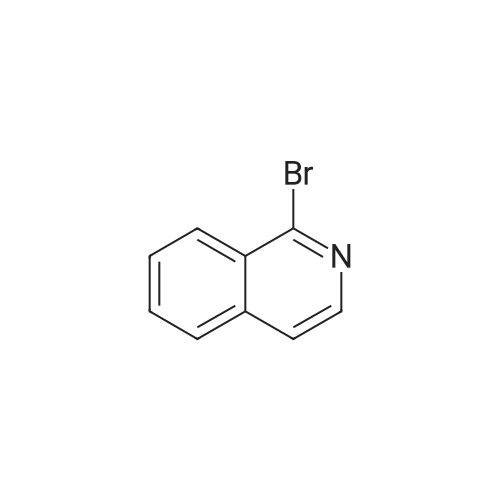
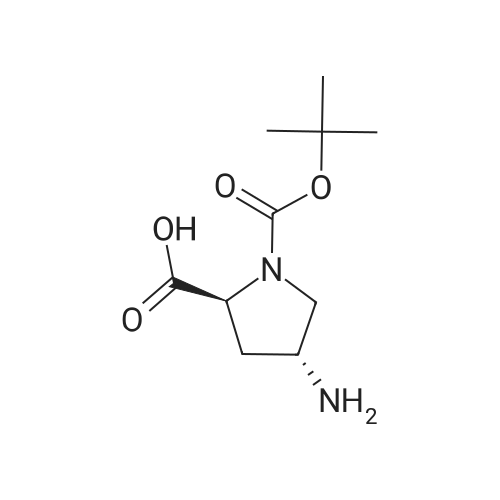
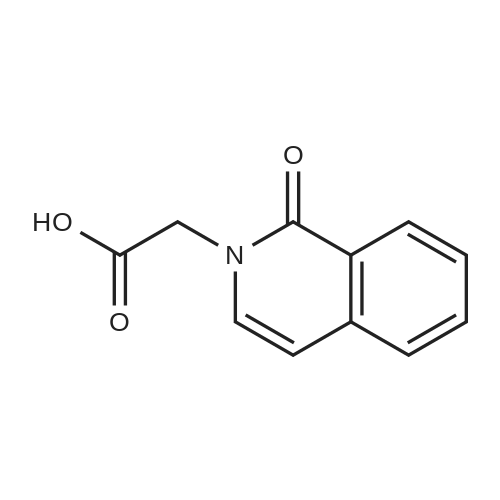
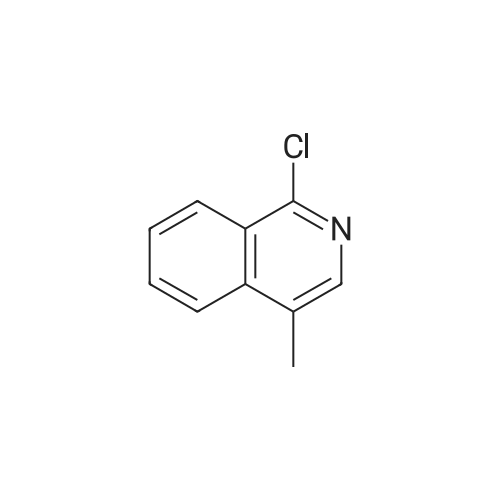
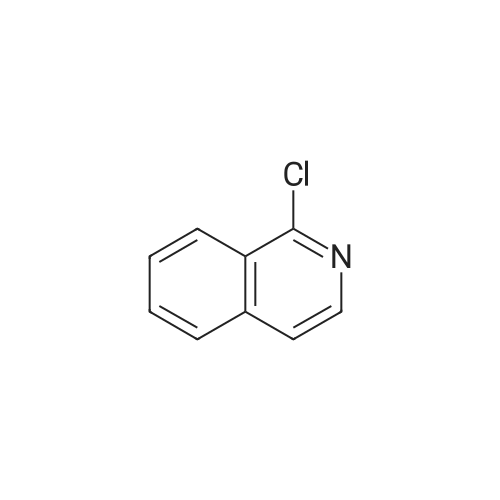
To a solution of (2S, 4R)Fmoc-4-amino-1-boc-pyrrolidine-2-carboxylic acid (400 mg, 0.884 mmol) in acetonitrile (15 mL), five drops of pyrrolidine was added. The reaction mixture was stirred at rt for 3hr. Then it was concentrated and put on high vacuum togive crude 4-amino-1-boc-pyrrolidine-2-carboxylic acid. In another round-bottomed flask, a solution ofPd2dba3 (40 mg, 5% mol) and racemic-BINAP (56 mg, 10% mol) was stirred under nitrogen in degassed toluene (8mL) at rt forlh. Then1-chloroisoquinoline (216 mg, 1.326 mmol) and sodium t-butoxide (340 mg,3.536 mmol) were added and the reaction mixture was stirred for 30 min. Then 4-amino-l-boc-pyrrolidine-2-carboxylic acid was added and the reaction mixture was heated under reflux for lh. Water was added to quench the reaction and the aqueous layer was separated and filtered through filter paper. It was then concentrated and purified by Prep. HPLC to give coupled product as TFA salt. (165 mg, 40% yield) 'H NMR(CD30D, 400 MHz)8 1.44 (m, 9H), 2.51-2. 74 (m, 2H), 3.64 (m,1H), 4.01 (m,1H), 4.49 (m,1H), 4.64 (m,1H), 7.30 (d, J=6.85 Hz,1H), 7.58 (d, J=6.85 Hz,1H), 7.79 (m,1H), 7.91-7. 99 (m, 2H), 8.56 (d, J=8. 56 Hz,1H). LC-MS (retention time: 1.707 min. ), MS m/z 358(MH+). |
|
|
To a solution of (2S,4R) Fmoc-4-amino-1-boc-pyrrolidine-2-carboxylic acid (400 mg, 0.884 mmol) in acetonitrile (15 mL), five drops of pyrrolidine was added. The reaction mixture was stirred at rt for 3hr. Then it was concentrated and put on high vacuum to give crude 4-amino-1-boc-pyrrolidine-2-carboxylic acid. In another round-bottomed flask, a solution of Pd2dba3 (40 mg, 5% mol)and racemic-BINAP (56 mg, 10% mol) was stirred under nitrogen in degassed toluene (8 mL) at rt for 1 h. Then 1-chloroisoquinoline (216 mg, 1.326 mmol) and sodium t-butoxide (340 mg, 3.536 mmol) were added and the reaction mixture was stirred for 30 min. Then 4-amino-1-boc-pyrrolidine-2-carboxylic acid was added and the reaction mixture was heated under reflux for 1 h. Water was added to quench the reaction and the aqueous layer was separated and filtered through filter paper. It was then concentrated and purified by Prep. HPLC to give coupled product as TFA salt. (165 mg, 40% yield) 1H NMR (CD3OD, 400 MHz) δ 1.44 (m, 9H), 2.51-2.74 (m, 2H), 3.64 (m, 1H), 4.01 (m, 1H), 4.49 (m, 1H), 4.64 (m, 1H), 7.30 (d, J=6.85 Hz, 1H), 7.58 (d, J=6.85 Hz, 1H), 7.79(m, 1H), 7.91-7.99 (m, 2H), 8.56 (d, J=8.56 Hz, 1H). MS m/z 358 (MH+). |
|
|
To a solution of (2S,4R) Fmoc-4-amino-1-boc-pyrrolidine-2-carboxylic acid (400 mg, 0.884 mmol) in acetonitrile (15 mL), five drops of pyrrolidine was added. The reaction mixture was stirred at rt for 3 hr. Then it was concentrated and put on high vacuum to give crude 4-amino-1-boc-pyrrolidine-2-carboxylic acid. In another round-bottomed flask, a solution of Pd2 dba3 (40 mg, 5% mol) and racemic-BINAP (56 mg, 10% mol) was stirred under nitrogen in degassed toluene (8 mL) at rt for 1 h. Then 1-chloroisoquinoline (216 mg, 1.326 mmol) and sodium t-butoxide (340 mg, 3.536 mmol) were added and the reaction mixture was stirred for 30 min. Then 4-amino-1-boc-pyrrolidine-2-carboxylic acid was added and the reaction mixture was heated under reflux for 1 h. Water was added to quench the reaction and the aqueous layer was separated and filtered through filter paper. It was then concentrated and purified by Prep. HPLC to give coupled product as TFA salt. (165 mg, 40% yield) 1H NMR (CD3OD, 400 MHz) δ 1.44 (m, 9H), 2.51-2.74 (m, 2H), 3.64 (m, 1H), 4.01 (m, 1H), 4.49 (m, 1H), 4.64 (m, 1H), 7.30 (d, J=6.85 Hz, 1H), 7.58 (d, J=6.85 Hz, 1H), 7.79 (m, 1H), 7.91-7.99 (m, 2H), 8.56 (d, J=8.56 Hz, 1H). MS m/z 358 (MH+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping