Alternatived Products of [ 19455-20-0 ]
Product Details of [ 19455-20-0 ]
| CAS No. : | 19455-20-0 |
MDL No. : | MFCD00058992 |
| Formula : |
C4H7KO2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | LBOHISOWGKIIKX-UHFFFAOYSA-M |
| M.W : |
126.20
|
Pubchem ID : | 23677462 |
| Synonyms : |
|
Application In Synthesis of [ 19455-20-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 19455-20-0 ]
- 1
-
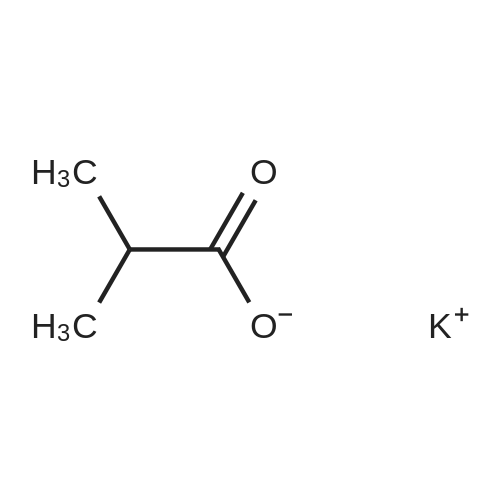 [ 19455-20-0 ]
[ 19455-20-0 ]

-
 [ 31951-90-3 ]
[ 31951-90-3 ]

-
 [ 21098-98-6 ]
[ 21098-98-6 ]
- 2
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
 [ 39074-38-9 ]
[ 39074-38-9 ]

-
 [ 40151-61-9 ]
[ 40151-61-9 ]
- 3
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
 [ 39074-64-1 ]
[ 39074-64-1 ]

-
 [ 40151-70-0 ]
[ 40151-70-0 ]
- 4
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
Methanesulfonic acid (2S,3R,4R)-4-(tert-butyl-dimethyl-silanyloxymethyl)-3,4-dimethyl-5-oxo-tetrahydro-furan-2-yl ester
[ No CAS ]

-
Isobutyric acid (2R,3R,4R)-4-(tert-butyl-dimethyl-silanyloxymethyl)-3,4-dimethyl-5-oxo-tetrahydro-furan-2-yl ester
[ No CAS ]

-
Isobutyric acid (2S,3R,4R)-4-(tert-butyl-dimethyl-silanyloxymethyl)-3,4-dimethyl-5-oxo-tetrahydro-furan-2-yl ester
[ No CAS ]
- 5
-
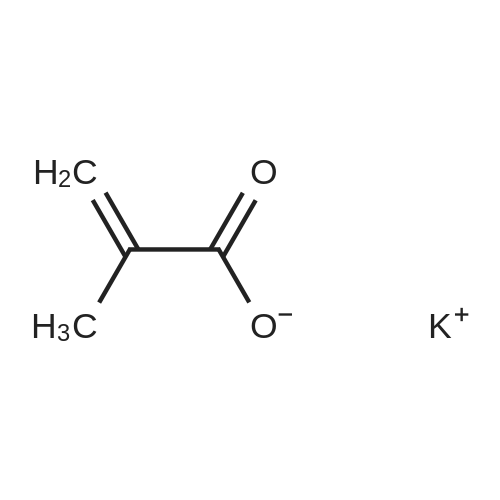 [ 6900-35-2 ]
[ 6900-35-2 ]

-
 [ 19455-20-0 ]
[ 19455-20-0 ]
- 6
-
 [ 144758-73-6 ]
[ 144758-73-6 ]

-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
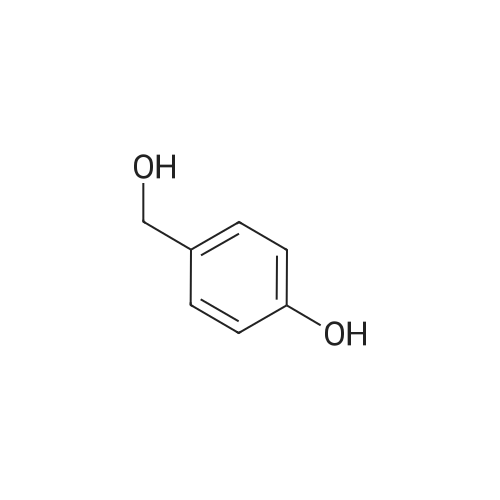 [ 623-05-2 ]
[ 623-05-2 ]

-
C14H18O7P(1-)*K(1+)
[ No CAS ]
- 7
-
 [ 144965-58-2 ]
[ 144965-58-2 ]

-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
 [ 623-05-2 ]
[ 623-05-2 ]

-
 [ 144965-64-0 ]
[ 144965-64-0 ]
- 8
-
potassium 1-methylethanecarboselenoate
[ No CAS ]

-
 [ 19455-20-0 ]
[ 19455-20-0 ]
- 9
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
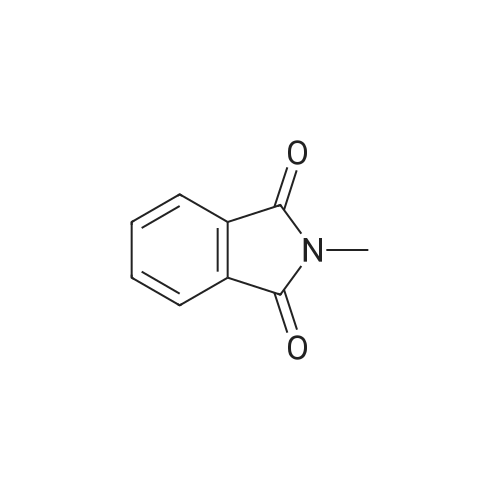 [ 550-44-7 ]
[ 550-44-7 ]

-
 [ 32360-86-4 ]
[ 32360-86-4 ]
- 11
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
 [ 6789-51-1 ]
[ 6789-51-1 ]

-
5-hydroxy-5-isopropyl-6-methyl-5,6-dihydropyrrolo[3,4-b]pyridine-7-one
[ No CAS ]

-
7-hydroxy-7-isopropyl-6-methyl-6,7-dihydropyrrolo[3,4-b]pyridine-5-one
[ No CAS ]
- 12
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
2-bromo-1-cyclohexyl-2-(3-fluoro-phenyl)-ethanone
[ No CAS ]

-
isobutyric acid 2-cyclohexyl-1-(3-fluoro-phenyl)-2-oxo-ethyl ester
[ No CAS ]
- 13
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
 [ 40222-71-7 ]
[ 40222-71-7 ]

-
 [ 107-21-1 ]
[ 107-21-1 ]

-
 [ 7440-66-6 ]
[ 7440-66-6 ]

-
 [ 21035-72-3 ]
[ 21035-72-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 91% |
With hydrogen sulfide; |
EXAMPLE 16 After heating to 90 C. a mixture of 32.24 g of cis-1,3-dibenzylhexahydrofuro[3,4-d]imidazole-2,4-dione, 7.75 g of <strong>[19455-20-0]potassium isobutyrate</strong>, 2.08 g of sulfur and 47.56 g of polyethylene glycol (the average molecular weight: 600), the mixture was stirred for 4.5 hours at the temperature while blowing 3.75 g of hydrogen sulfide at a rate of 10 ml/minute. Then blowing of hydrogen sulfide was stopped and the resultant mixture was stirred for 3.5 hours at the temperature. Thereafter, the resulting mixture was subjected to reduction reaction with zinc powder and after-treatment as described in Example 1, obtained oil layer was subjected to LC analysis. The net yield of cis-1,3-dibenzylhexahydrothieno[3,4-d]imidazole-2,4-dione was 30.93 g (yield percentage: 91%). |
- 14
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
α-Ethoxy-ω-hydroxy-poly (1,3-dioxolane)
[ No CAS ]

-
 [ 105-36-2 ]
[ 105-36-2 ]

-
α-ethoxy-ω-carboxymethyl-poly(1,3-dioxolane)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium chloride; In sodium hydroxide; cyclohexane; toluene; |
C. Succinimidyl α-Ethoxy-ω-carboxymethyl-poly (1;3-dioxolane) α-Ethoxy-ω-hydroxy-poly(1,3-dioxolane) (5 g, 0.09 mmole) was dissolved in anhydrous toluene (20 mL) and potassium t-butanoate (1.6 g, 14 mmole) was added. The solution was brought to reflux, then kept at 50 C. for 5 h. Ethyl bromoacetate (1.6 mL, 14 mmole) was slowly added and the solution was stirred overnight at the same temperature. The precipitated salts were removed by filtration and washed with methylene chloride (20 mL). The polymer was recovered by partially concentrating the filtrate and slowly pouring into ether/cyclohexane (1:1, 200 mL). The polymer was dried in vacuo, then dissolved in 1N NaOH (20 mL) and NaCl (4 g) was added. After 1 hour, this solution was acidified with 2N HCl to pH 3.0 and extracted with dichloromethane (3*50 mL). The combined organic phase was dried (MgSO4), concentrated to 30 mL, and poured into cool ether/cyclohexane (3:1, 300 mL). The precipitate was collected by filtration and dried in vacuo. The resulting α-ethoxy-ω-carboxymethyl-poly(1,3-dioxolane), (5 g, 0.09 mmole) was dissolved in anhydrous dichloromethane (20 mL), and N-hydroxysuccinimide (0.23 g, 2.0 mmole) and dicyclohexylcarbodiimide (0. 413 g, 2.00 mmole) were added. |
- 15
-
 [ 79-31-2 ]
[ 79-31-2 ]

-
 [ 19455-20-0 ]
[ 19455-20-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium hydroxide; In ethanol; |
E. Potassium 2-methylpropanoate A mixture of 8.8 g of 2-methylpropanoic acid and 5.6 g of potassium hydroxide in 25 ml of ethanol was allowed to react for 45 minutes. The solution was diluted to a final volume of 500 ml with diethyl ether. The precipitated salt was collected and dried to yield 9.4 g of potassium-2-methylpropanoate. |
- 16
-
 [ 19455-20-0 ]
[ 19455-20-0 ]

-
N'-(4-cyano-3-isopropyl-5-isothiazolyl)-N,N-dimethylchloroformamidine
[ No CAS ]

-
 [ 63940-90-9 ]
[ 63940-90-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In acetonitrile; |
F. N'-(4-Cyano-3-isopropyl-5-isothiazolyl)-N,N-dimethyl-N'-(2-methylpropanoyl)urea A mixture of 5 g N'-(4-cyano-3-isopropyl-5-isothiazolyl)-N,N-dimethylchloroformamidine (from part D), 4.8 g of potassium 2-methylpropanate (from part E), 20 ml of dry acetonitrile and a catalytic amount of 2,3,11,12-dibenzo-1,4,7,10,13,16-hexaoxacycloctadeca-2,11-diene (dibenzo-18-crown-6) was allowed to react during 24 hours. The inorganic salts were removed by filtration. The volatile materials were evaporated under reduced pressure. The residue was recrystallized from 150 ml of 70:30 hexane:benzene to yield 3.3 g of N'-(4-cyano-3-isopropyl-5-isothiazolyl)-N,N-dimethyl-N'-(2-methylpropanoyl)urea, mp 127-129. The ir and nmr spectra were consistent with the assigned structure. The structure assignment was confirmed by the uv spectrum. Analysis: Calc'd for C14 H20 N4 O2 S: C, 54.52; H, 6.59; N, 18.17; S, 10.40; Found: C, 54.81; H, 6.69; N, 17.93; S, 10.10. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










