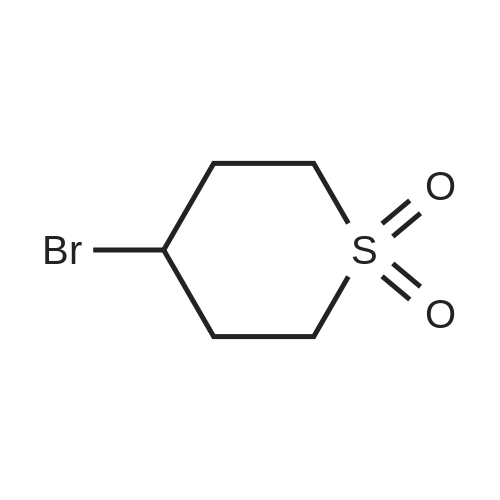
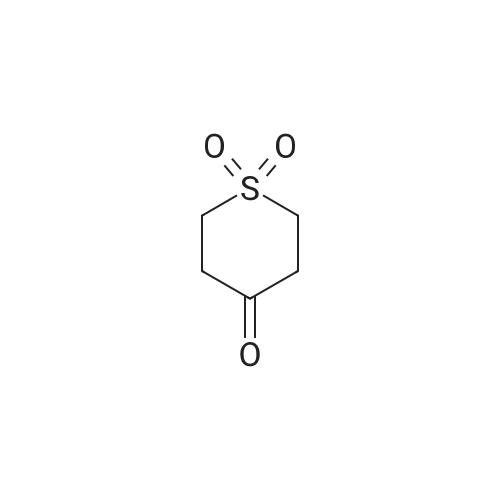
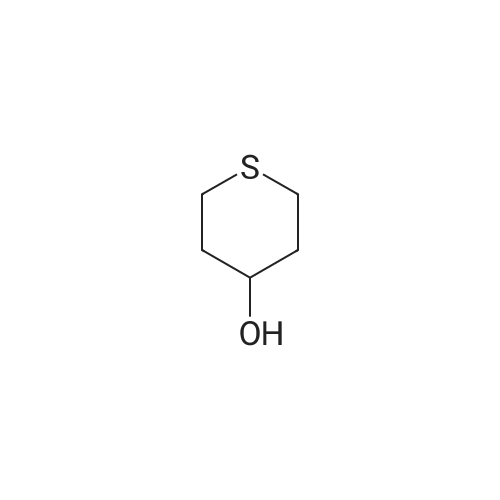
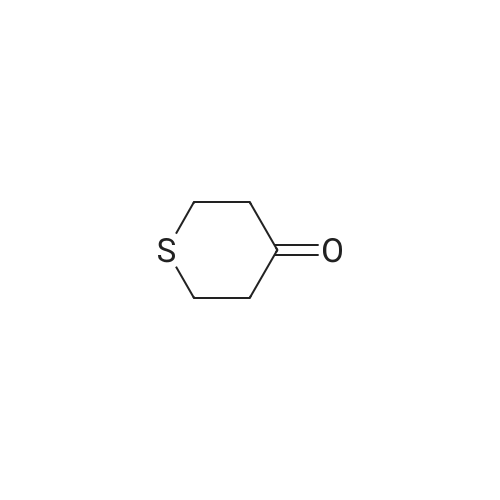
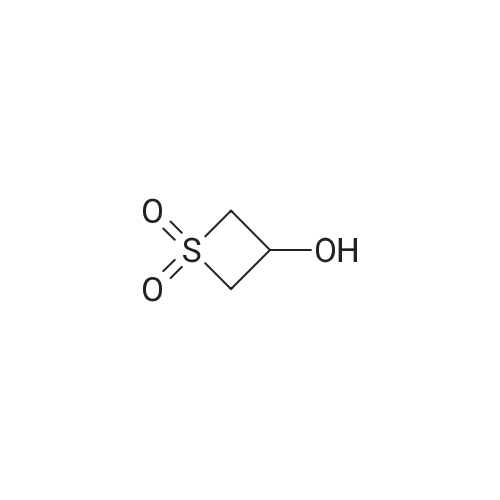
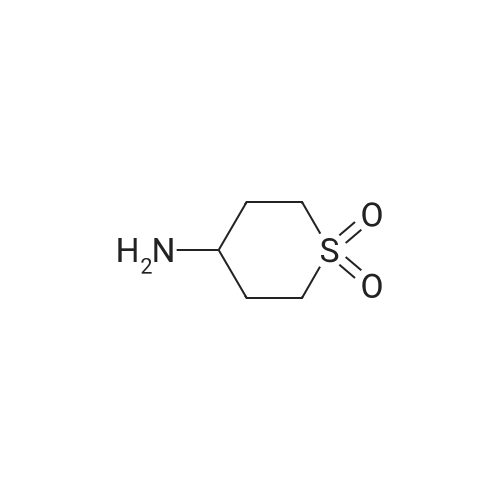
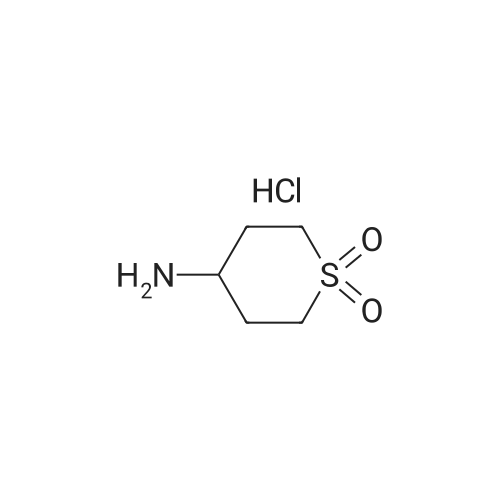
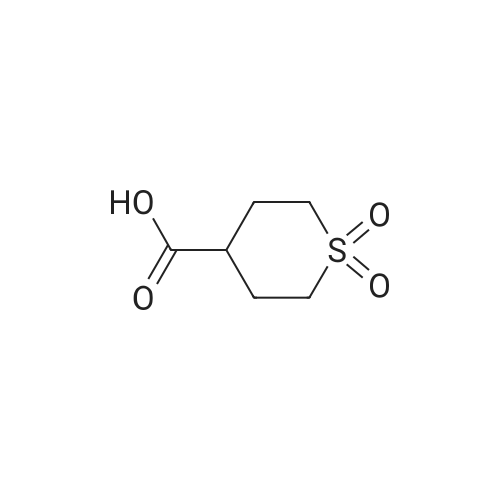
| 95% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 3.16667h; |
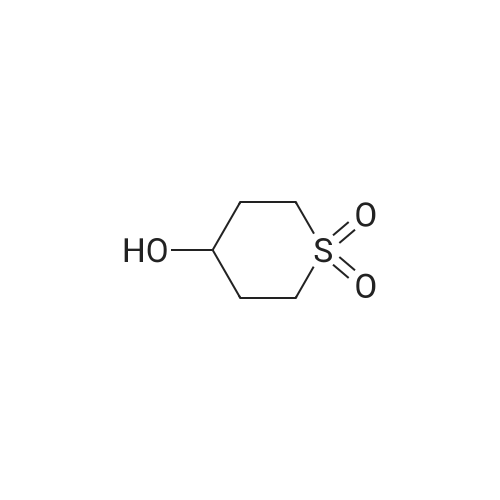
Step 1. l,l-dioxidotetrahydro-2H-thiopyran-4-yl methanesulfonate [0705] Triethylamine (7.80 mL, 55.9 mmol) was added to a solution of 4-hydroxytetrahydro-2H- thiopyran 1,1-dioxide (3.5 g, 23.3 mmol) in dichloromethane (35.0 mL). The reaction solution was cooled to 0 °C and methanesulfonyl chloride (3.25 ml, 41.9 mmol) was added. After 10 minutes, the reaction solution was warmed to room temperature and stirred for 3 h. The reaction was quenched via the addition of saturated aqueous ammonium chloride solution (15 mL). The organic layer was separated and washed with saturated aqueous sodium bicarbonate solution (15 mL) and brine (15 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to afford an off-white solid. The solid residue was suspended in ethyl acetate (20 mL) and filtered. The filtered solid was then collected and dried in vacuo affording 1 , 1 -dioxidotetrahydro-2H-thiopyran-4-yl methanesulfonate (5.1 g, 95percent) as a white solid. 1H NMR (400 MHz, CDC13) delta ppm 2.38 - 2.56 (m, 4 H) 2.94 - 3.06 (m, 2 H) 3.10 (s, 3 H) 3.23 - 3.39 (m, 2 H) 5.03 (tt, J=4.74, 2.49 Hz, 1 H) |
| 95% |
With triethylamine; In dichloromethane; at 0 - 20℃; for 3.16h; |
Triethylamine (7.80 mL, 55.9 mmol) was added to a solution of 4-hydroxytetrahydro- 2H-thiopyran 1,1-dioxide (3.5 g, 23.3 mmol) in dichloromethane (35.0 mL). The reaction solution was cooled to 0 °C and methanesulfonyl chloride (3.25 ml, 41.9 mmol) was added. After 10 minutes, the reaction solution was warmed to room temperature and stirred for 3 h. The reaction was quenched via the addition of saturated aqueous ammonium chloride solution (15 mL). The organic layer was separated and washed with saturated aqueous sodium bicarbonate solution (15 mL) and brine (15 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to afford an off-white solid. The solid residue was suspended in ethyl acetate (20 mL) and filtered. The filtered solid was then collected and dried in vacuo affording 1,1-dioxidotetrahydro-2H-thiopyran-4-yl methanesulfonate (5.1 g, 95percent) as a white solid.1H NMR (400 MHz, CDCl3) delta ppm 2.38 - 2.56 (m, 4 H) 2.94 - 3.06 (m, 2 H) 3.10 (s, 3 H) 3.23 - 3.39 (m, 2 H) 5.03 (tt, J = 4.74, 2.49 Hz, 1 H) |
| 75% |
With triethylamine; In dichloromethane; at 20℃; |
A 250-mL round-bottom flask was charged with <strong>[194152-05-1]4-hydroxy-thiane-1,1-dione</strong> (3.50 g, 23.3 mmol, 1.00 equiv), methanesulfonyl chloride (5.30 g, 46.5 mmol, 2.00 equiv), triethylamine (7.10 g, 70.2 mmol, 3.00 equiv), and DCM (40 mL). The resulting solution was stirred overnight at room temperature and then quenched with water (40 mL). The resulting mixture was extracted with DCM (3 x 30 mL) and the organic layers were combined, washed with brine (1 x 200 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was chromatographed on a silica gel column to provide 4.00 g (75percent yield) of 1,1 -dioxo-thian-4-yl methanesulfonate as a white solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping