| 94% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; In dichloromethane; at 20℃; for 30h;Product distribution / selectivity; |
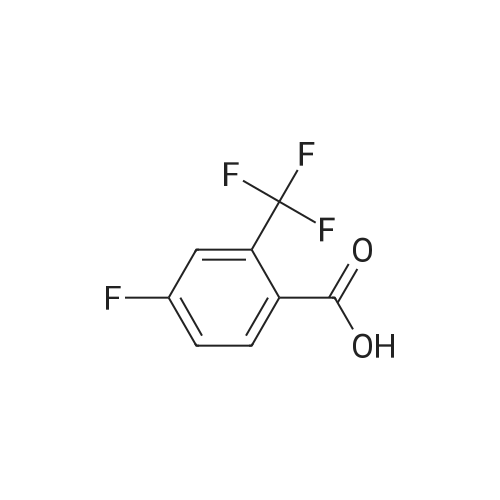
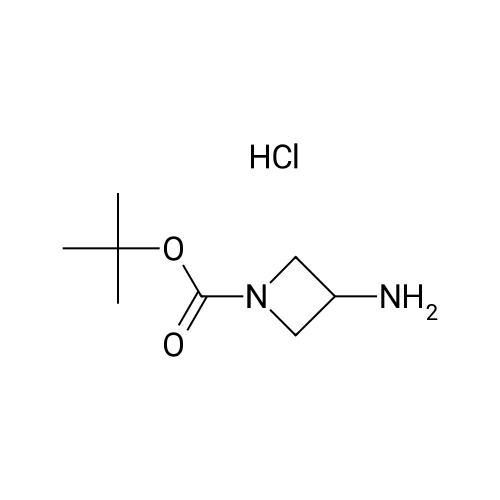
compound/ Purity d wt. or reagent name (%) MW (g/mL) eq. mol vol. 3 99.0 247.17 1.00 1.00 2.40 600.0 g N-Boc-3- 98.00 172.23 1.38 1.06 2.55 448.0 g (amino) azetidine (4) HOBT 98.00 135.13 1.00 0.117 0.28 37.7 g EDCl 99.00 191.70 1.02 1.42 3.41 660.0 g Tri- 99.0 101.19 0.73 1.30 3.13 0.44 L ethylamine Di- 99.0 84.93 1.32 135.91 8.8 L chloro- methane To a 22-L 4-neck round bottom flask equipped with a thermocouple controller, mechanical stirrer, heating mantle, condenser and a nitrogen inlet adapter was charged with acid 3 as prepared in the previous step (600 g; 2.40 mol), dichloromethane (8.8 L), TEA (440 mL; 3.13 mol), N-Boc-3-(amino)azetidine (4, CNH Technologies, Inc.) (448 g; 2.55 mol) and HOBT (37.7 g; 0.28 mol, AK Scientific), and the mixture was stirred at 20 C. for 5 min, EDCI (506 g; 2.64 mol; 1.1 eq., AK Scientific) was added as one portion. The mixture was stirred at 20 C. for an additional 6 h. More EDCI (120.0 g, 0.626 mol; 0.26 eq) was added and the reaction was stirred at 20 C. for 20 h. EDCI (33.3 g; 0.174 mol; 0.05 eq.) was added again at the 21st h and the reaction was stirred at 20 C. for an additional 4 h. The reaction was washed with saturated NaHCO3 (4 L×2) (aqueous pH=9) and brine (3 L×2), and the separated organic phase was concentrated at 60 C. under house vacuum (120 mmHg) and then high vacuum (20 mmHg) to give crude 5 as a yellowish think syrup.To a 22-L 4-neck round bottom flask equipped with a thermocouple controller, mechanical stirrer, heating mantle, condenser and a nitrogen inlet adapter was charged a warm solution (60 C.) of crude 5 in EtOAc (3.1 L) and the mixture was heated to 73 C. with stirring, while warm (60 C.) heptane (10.0 L) was added as five portions (2 L×5) over 30 min. The resulting slightly white turbid solution was stirred at 73 C. for 10 min. The heating mantle was removed and the mixture was gradually cooled to 20 C. over 3 h, and further to 10 C. in a water bath and stirred for 1 h. The solid was collected by filtration, washed with hexanes (300 mL×2), dried under air-suction and then placed in a drying oven at 50 C. under house vacuum for 20 h to afford pure title compound, 5, as an off-white crystalline solid. The structure of 5 was confirmed by 1H-NMR. |
| 80% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃; for 4h; |
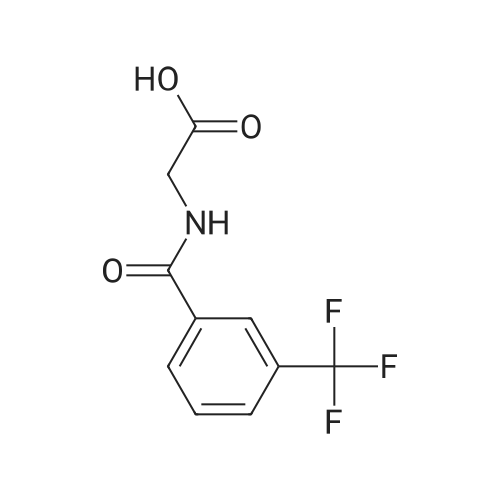
General procedure: N-(Azetidin-3-ylcarbamoylmethyl)-3-trifluoromethyl-benzamide TFA salt: 3-Amino-azetidine-1-carboxylic acid tert-butyl ester (1.2 g, 6.97 mmol) and <strong>[17794-48-8](3-trifluoromethyl-benzoylamino)-acetic acid</strong> (1.57 g, 6.36 mmol) were treated with EDCI (1.57 g, 6.36 mmol), HOBT (1.22 g, 6.36 mmol) in DCM (10 mL) at room temperature for 4 hours. The reaction solution was partitioned between DCM and water. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated to give yellow oil, which was then purified by silica gel column on a CombiFlash system using hexanes and ethyl acetate (from 10% ethyl acetate to 100% ethyl acetate) to afford 3-[2-(3-trifluoro-methyl-benzoylamino)-acetylamino]-azetidine-1-carboxylic acid tert-butyl ester as white solid, 2.23 g, 80% yield. 1H NMR (400 MHz, CDCl3) d 8.10 (s, 1H), 8.02 (d, J = 6.6 Hz, 1H), 7.80 (d, J = 6.8 Hz, 1H), 7.56 (t, J = 6.5 Hz, 1H), 4.61 (m, 1H), 4.25 (t, J = 7.2 Hz, 2H), 4.18 (d, J = 5.5 Hz, 2H), 3.82 (t, J = 7.5 Hz, 2H), 1.41 (s, 9H). 3-[2-(3-Trifluoromethyl-benzoylamino)-acetylamino]-azetidine-1-carboxylic acid tert-butyl ester (2.10 g, 5.24 mmol) was dissolved in 1:1 TFA and DCM mixed solution (10 mL) at room temperature. The reaction was stirred for another 2 hours. The solvent was removed and the residue was dried to give N-(azetidin-3-ylcarbamoylmethyl)-3-trifluoromethyl-benzamide as a TFA salt containing extra TFA (colorless oil), ~ 2.5 g, 100% yield. 1H NMR (400 MHz, CDCl3) d 8.10 (s, 1H), 8.05 (d, J = 6.0 Hz, 1H), 7.78 (d, J = 6.2 Hz, 1H), 7.55 (m, 2H), 4.78 (m, 1H), 4.15 (d, J = 3.2 Hz, 2H), 3.95 (t, J = 7.0 Hz, 2H), 3.52 (t, J = 7.0 Hz, 2H).8-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1,4-dioxa-spiro[4.5]dec-7-ene (PCT Int. Appl. WO2006064189, 0.292 g, 1.10 mmol), 5-bromobenzo[d][1,3]dioxole (Aldrich, 161 mg, 0.801 mmol), and tetrakis (triphenylphosphino)palladium(0) (Aldrich, 0.048 g, 0.042 mmol) were dissolved in 1,4-dioxane (9 mL), treated with 2M aqueous Na2CO3 (2.0 mL, 4.0 mmol), bubbled with argon for a few minutes, and heated to 100oC under reflux condenser for 24 h. After cooling to ambient temperature, the reaction was diluted with water (30 mL), extracted thrice with dichloromethane, aqueous layer acidified to ca. pH 7, extracted twice more with dichloromethane, and the combined organic layers washed with brine, dried over Na2SO4, filtered, and the filtrate concentrated in vacuo to give an orange oil. This was purified by thin layer chromatography on silica gel (EtOAc) to give 5-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)benzo[d][1,3]dioxole as a yellow solid (110 mg, 53%). 5-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)benzo[d][1,3]dioxole (110 mg, 0.42 mmol) in MeOH (5 mL) was placed in a hydrogenation Par Shaker under 40 psi at room temperature using 5% Pd/C (~ 50 mg) as catalyst for 2 hour. The resulting solution was filtered through a pad of Celite , concentrated and purified by silica gel column on a CombiFlash system to afford 5-(1,4-dioxaspiro[4.5]decan-8-yl)benzo[d][1,3]dioxole as white solid, 91 mg, 82% yield. 5-(1,4-dioxaspiro[4.5]decan-8-yl)benzo[d][1,3]dioxole (91 mg, 0.35 mmol) was treated with 1N HCl ( ~ 2 mL) in acetone (4 mL) at room temperature for 4 hours. The reaction was neutralized with saturate NaHCO3 solution and the solvent was removed. The residue was partitioned between ethyl acetate and water. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated to give yellow solid, which was then purified by silica gel column on a CombiFlash system using hexanes and ethyl acetate (from 10% ethyl acetate to 100% ethyl acetate) to afford 4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone as white solid, 75 mg, 98% yield.4-(benzo[d][1,3]dioxol-5-yl)cyclohexanone (75 mg, 0.35 mmol) and N-(azetidin-3-ylcarbamoylmethyl)-3-trifluoromethyl-benzamide TFA salt (151 mg, 0.50 mmol) in DCM (2 mL) was treated with TEA (0.1 mL, 0.75 mmol) for 10 min followed by NaBH(OAc)3 (Aldrich, 225 mg, 1.05 mmol) for another 4 hours at room temperature. The reaction was quenched with saturated sodium bicarbonate. The organic layer was separated and the aqueous layer was extracted 3 times with chloroform and IPA "cocktail" (~ 3:1, v/v). The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated to give the crude product, which was then purified by a CombiFlash system using ethyl acetate and 7N NH3 in MeOH as eluent (from pure ethyl acetate to 5% 7N NH3 in MeOH in ethyl acetate) to afford two title compounds as white solids: 8a, less polar isomer (cis), 67 mg, 38% yield; 9a, more polar isomer (trans), 45 mg, 26% yield. |
|
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20℃; for 4h; |
A solution of 3-amino-azetidine-1-carboxylic acid tert-butyl ester (BetaPharma, 1.2 g, 6.97 mmol) and <strong>[17794-48-8](3-trifluoromethyl-benzoylamino)-acetic acid</strong> (Bionet, 1.57 g, 6.36 mmol) in DCM (10 mL) was treated with EDCI (Aldrich, 1.57 g, 6.36 mmol) and HOBT (Aldrich, 1.22 g, 6.36 mmol) at room temperature for 4 hours. The reaction solution was partitioned between DCM and water. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated to give a yellow oil, which was then purified by silica gel column on a CombiFlash system using hexanes and ethyl acetate (from 10% ethyl acetate to 100% ethyl acetate) to afford the title compound as a white solid. 1H NMR (400 MHz, CDCl3) delta 8.10 (s, 1H), 8.02 (d, J=6.6 Hz, 1H), 7.80 (d, J=6.8 Hz, 1H), 7.56 (t, J=6.5 Hz, 1H), 4.61 (m, 1H), 4.25 (t, J=7.2 Hz, 2H), 4.18 (d, J=5.5 Hz, 2H), 3.82 (t, J=7.5 Hz, 2H), 1.41 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping