| 86% |
With dmap; dicyclohexyl-carbodiimide; In dichloromethane; |
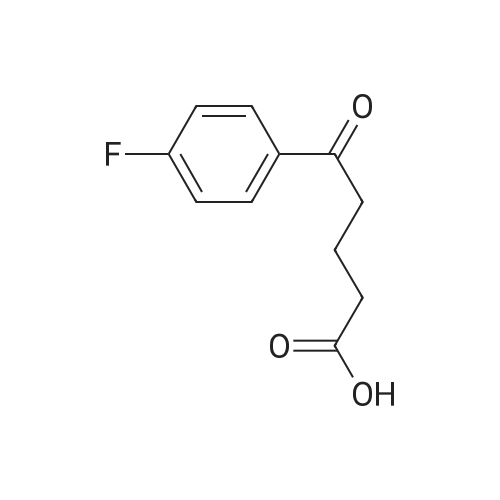
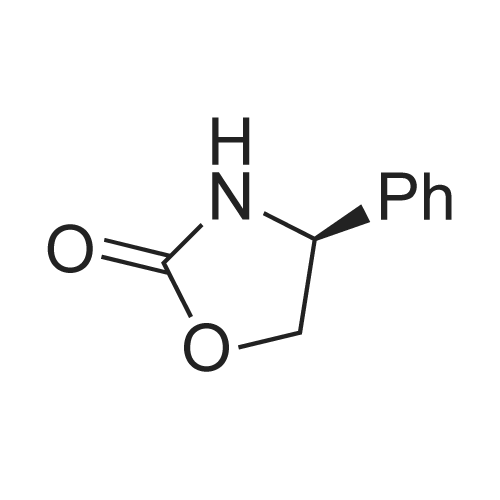
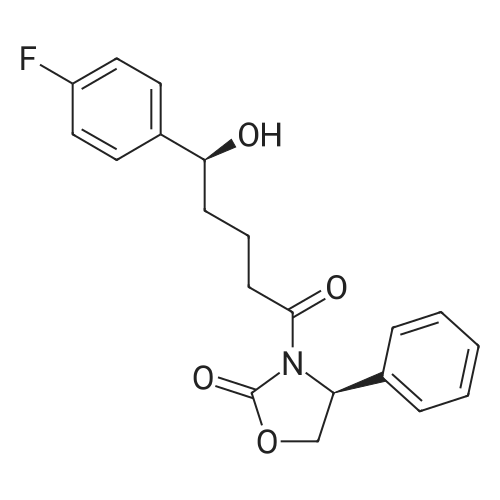
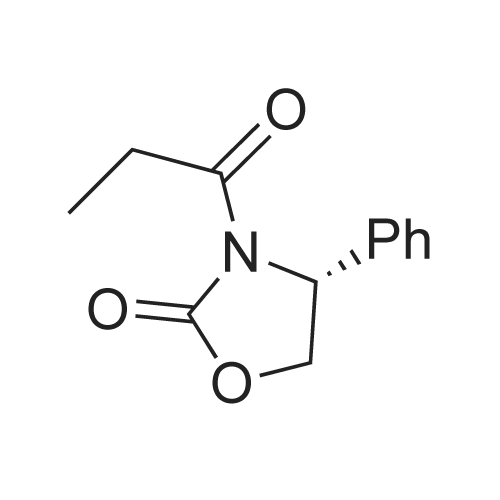
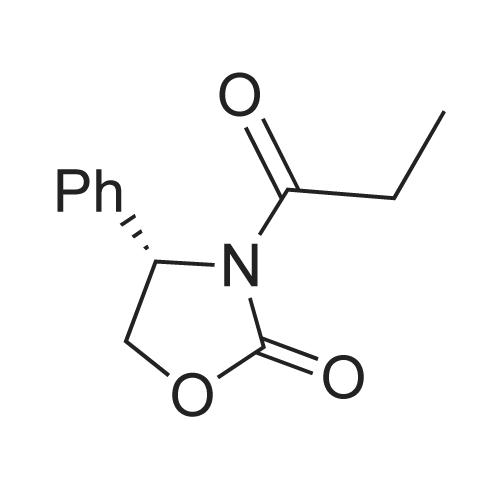
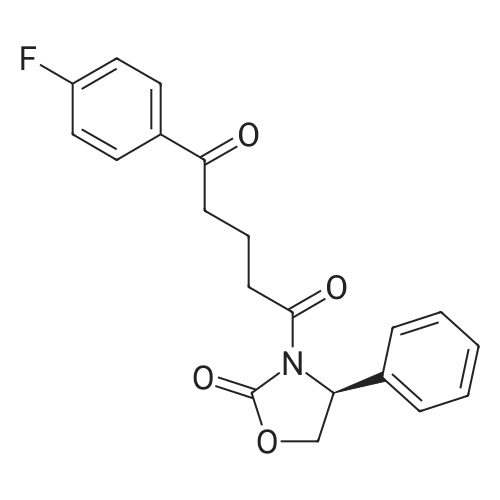
Example 1: Preparation of ezetimibe; (1-1) Preparation of 3-[5-(fluorophenyl)-l,5-dioxapentyl]-4(S)- phenyl-2-oxazolidinone (Formula 7); 200 g of <strong>[149437-76-3]5-(4-fluorophenyl)-5-oxopentanoic acid</strong> of formula 8, 16O g of (S)-4-phenyloxazolidine-2-one of formula 9, and 11.6 g of 4-dimethylaminopyridine were dissolved in 600 m£ of dichloromethane to prepare a reaction mixture. A solution which was prepared by dissolving 157 g of N,N'-dicyclohexylcarboimide in 200 ml of dichloromethane was added to the reaction mixture and stirred for 2 hours. After completion of the reaction, the resulting reaction mixture was filtered to remove by-products. The filtrate thus obtained was washed successively with 1 I of 6N HCl, 1 ? of water, and 1 ? of saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered, and distilled under a reduced pressure to remove the solvent. The residue thus obtained was dissolved in 2 I of methanol by heating and cooled to induce crystallization. 2 £ of water was added thereto and stirred for 30 min. The solid thus obtained was isolated by filtering to obtain 289 g of the title compound as a white solid (yield: 86%).1H NMR(300MHz, CDCl3) : delta 7.92 (2H, M), 7.35-7.13 (5H, m), 7.04 (2H, m), 5.43 (IH, q), 4.75(1H, t), 4.22 (IH, q), 3.05-2.93 (4H, m), 2.03 (2H, m) |
| 85.7% |
|
5-(4-Fluorophenyl)-5-oxopentanoic acid (21.02 g, 100.0 mmol) and 4 dimethylamino- pyridine (16.25 g, 133.0 mmol) were dissolved in N,N-dimethylformamide (100 mL, 1.0 M) to afford a copious white precipitate suspended in solution. The reaction was cooled to 2 0C (ice/water bath), and trimethylacetyl chloride (16.40 mL, 16.04 g, 133.0 mmol) was added drop-wise to afford a pale yellow mixture. The rate of addition was controlled in order to keep the temperature at or below 5 0C. A heavy white precipitate was formed and the mixture was allowed to warm to room temperature and stirred for 1.5 h. The mixture was charged with (6)-(+)-4-phenyl-2- oxazolidinone (16.32 g, 100.0 mmol) and 4-dimethylaminopyridine (12.22 g, 100.0 mmol) both as solids to afford a yellow colored suspension. The reaction was stirred at 30 C - 35 0C for 2 h. An aliquot was removed for analysis by TLC and HPLC. The pale olive colored suspension was poured into water (400 mL) while stirring vigorously and cooling the mixture in an ice-brine bath, transferred with water (150 mL) and stirred with ice-cooling for 1.5 h to afford a solution with an off-white precipitate. The compound was filtered, transferred with water (2 x 25 mL), washed with water (50 mL) and air dried for 15 min to afford an off-white moist clumpy powder. The material was crystallized from isopropanol (58.0 mL; 1.6 mL/g theoretical yield) by heating to near reflux to afford a golden yellow colored solution. The solution was cooled slowly to room temperature over 12 h, a seed crystal was added and crystals began to precipitate. The mixture was cooled in an ice/water bath and stirred for 1 h. The crystals were filtered, transferred with cold isopropanol (2 x 10 mL), washed with cold isopropanol (25 mL), air dried and vacuum dried to constant weight to afford (45)-4-phenyl-3-[5-(4-fluorophenyl)-5-oxopentanoyl]-l,3- oxazolidin-2-one (30.46 g, 85.7 % yield) as a white crystalline solid; m.p. 91.0 0C; EPO <DP n="43"/>R/ 0.40 (1:2 ethyl acetate-hexane); HPLC Rr7.02 min; HPLC purity 94 %. 1H NMR (300 MHz, CDCl3) delta 7.93 (dd, J= 5.4, 9.0 Hz, 2H), 7.28-7.42 (m, 5H), 7.10 (dd, J= 8.5, 9.0 Hz, 2H), 5.43 (dd, J= 3.7, 8.7 Hz, IH), 4.70 (t, J= 8.9 Hz, IH), 4.28 (dd, J= 3.7, 8.7 Hz, IH), 3.05 (dt, J= 1.2, 7.3 Hz, 2H), 2.97 (t, J= 7.3, 2H), 2.05 (p, J= 7.3 Hz, 2H), ppm. |
| 85% |
|
To the soulution of compound 2 (700 g, 3.33 mol, 1.1 eq) dissolved in THF (5 L) was added triethylamine (1.2 L, 7.87 mol, 2.6 eq) at room temperature under N2 atmosphere. Then the solution of pivalic acid chloride (400 g, 3.33 mol, 1.1 eq) in THF (700 mL) was added dropwise to the reaction mixture cooled to -10 C for about 2 h. After addition, the mixture was stirred for 2 h at this temperature. TLC showed the compound 2 was consumed up, then the Evans auxiliary (493.5 g, 3 mol, 1 eq) and anhydrous LiCl (150.5 g, 3.5 mol,1.17 eq) were added sequencely. The mixture was warmed to room temperature and stirred for 2 h till the anhydride intermediate was consumed up. Then ethylacetate (2 L) and NH4Cl aq (1 L) were added, stirred for 15 min, stayed and separated, the aqueous layer was extracted with ethyl acetate (500 mL×2), combined the organic phase, washed with water (250 mL ×2), concentrated.The residue was recrystallized from isopropanol (7 L) to afforded compound 4 as a white solid (913.6 g, 85.0% yield). HPLC purity: 99.2%, mp: 94-95 C [lit.9(b) 91C]. 1H-NMR (CDCl3,400 MHz): delta 7.94-7.93 (m, 2H, J=2), 7.40-7.29 (m, 5H), 7.10-7.01 (m, 2H), 5.45-5.41 (m, 1H), 4.72-4.63 (m, 1H), 4.30-4.27 (m, 1H), 3.06 (t, 2H, J=8), 2.97 (t, 2H, J=12), 2.10-2.03 (m, 2H). 13C-NMR (CDCl3, 100MHz): delta 197.7, 172.2, 167.0, 164.4, 153.8, 139.2, 133.3, 130.7, 130.6, 129.2, 128.7, 126.0, 115.7, 115.5, 70.1, 57.6, 37.3, 34.8, 18.5. MS (ESI) m/z calcd. for C20H18FNO4: 355.12, calcd. for C20H18FNO4Na (M+Na)=378.12, found 378.04 (M+Na). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping