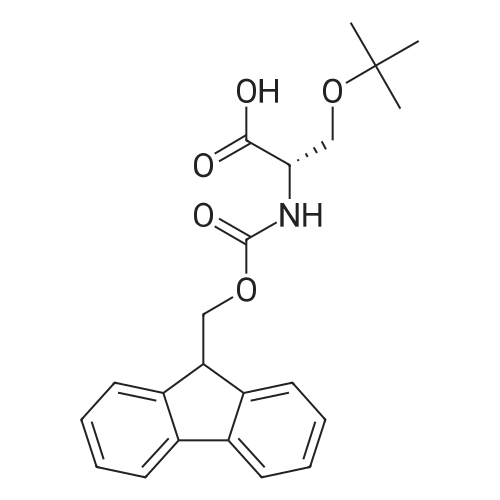
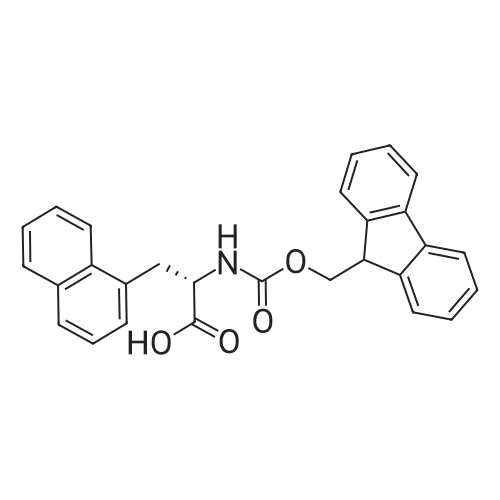
|
|
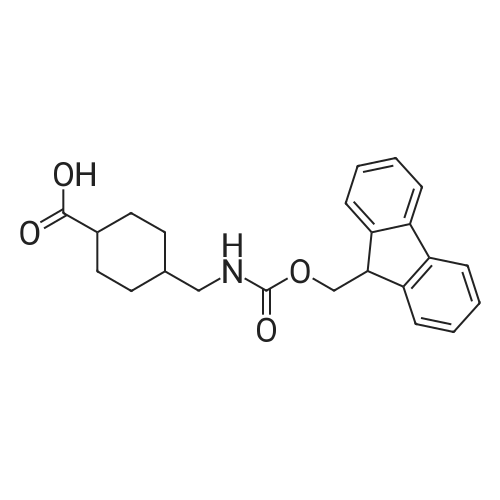
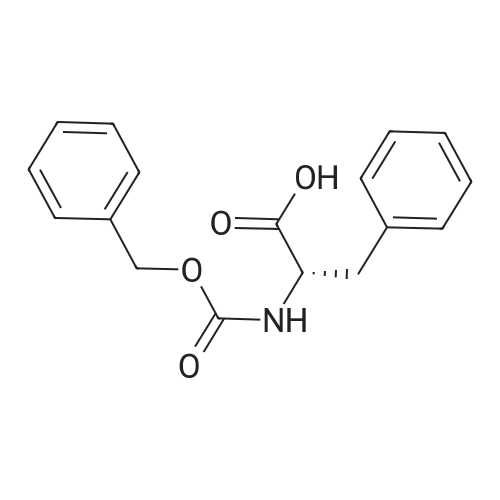
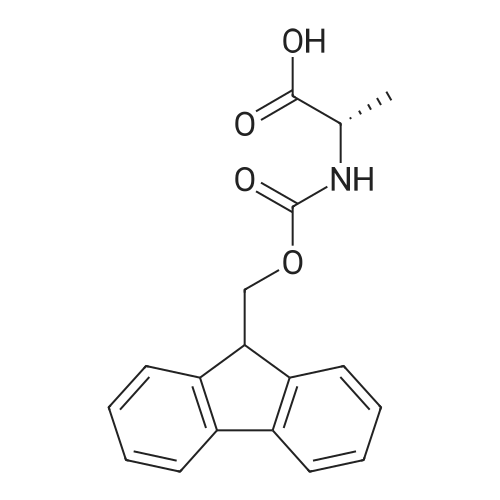
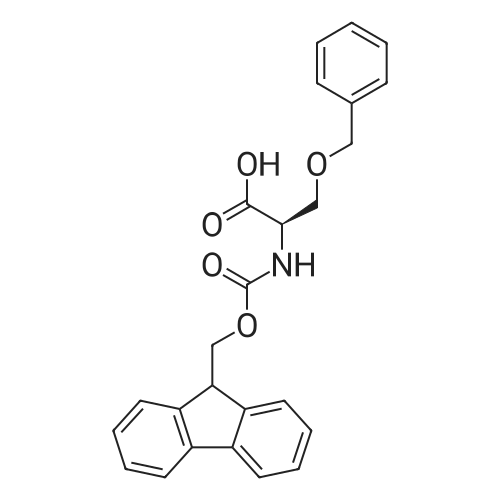
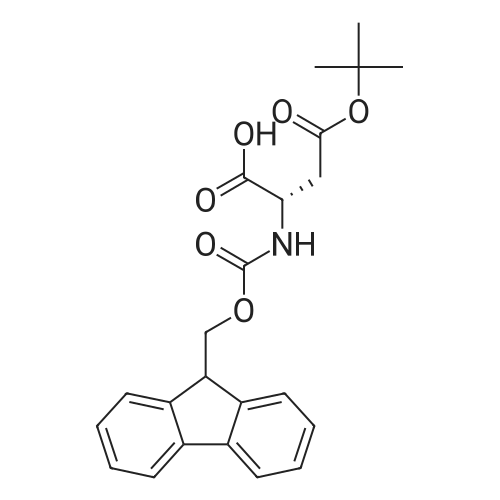
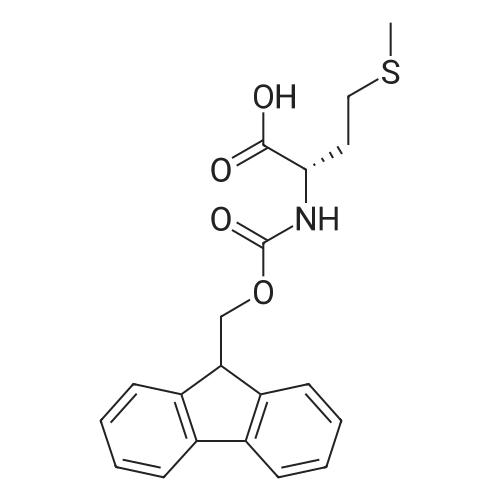
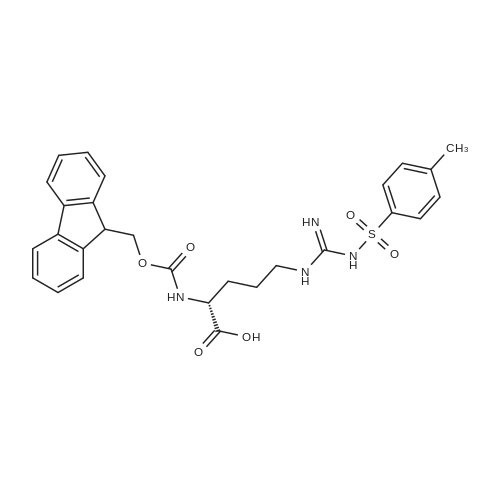
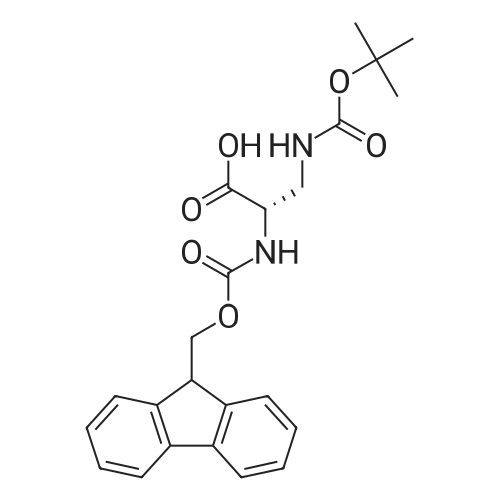
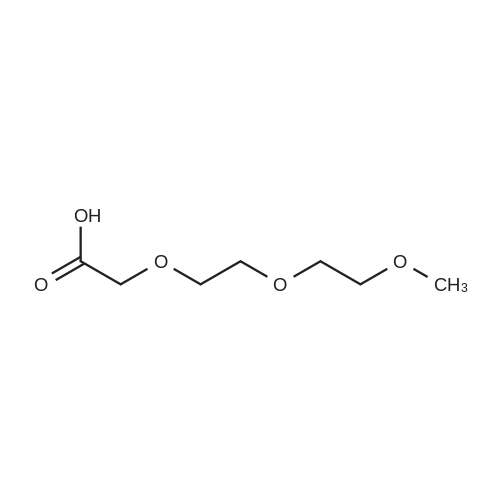
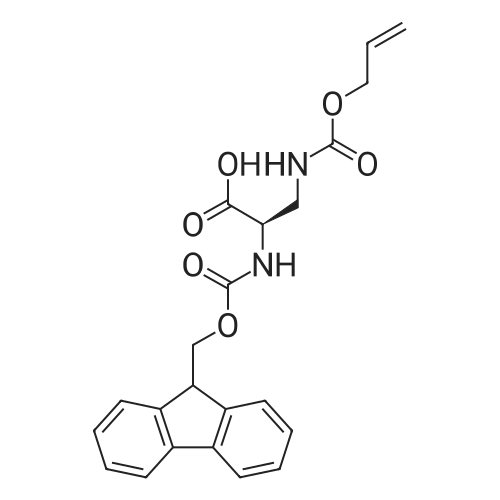
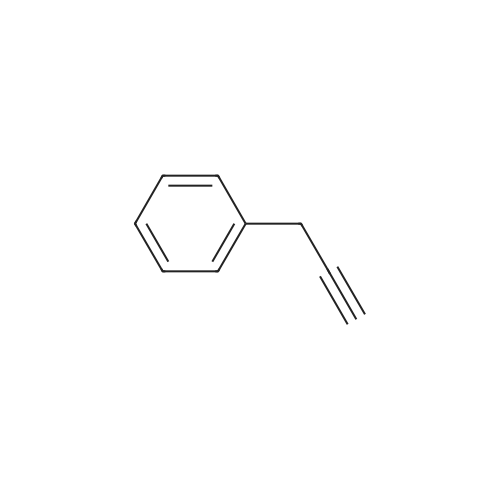
General procedure: A solution of piperidine (1 mL) in anhydrous DMF (2 mL) and DCM (2 mL) was added to the peptide vessel containing the swelled resin (0.1 mmol). The mixture was allowed to rock for 30 minutes at room temperature. The solution was drained, and the resin was rinsed with DMF (3 mL x 2). A solution of 1:1 DCM/DMF (3 mL x 2) was added to the resin and the peptide vessel was allowed to rock for 3 minutes. The solution was drained, and DMF (3 mL) was added. The vessel was rocked for 3 minutes, and the solvent was drained. General procedure for peptide synthesis:1 Fmoc-amino acid (0.5 mmol), HOBT (0.068 g, 0.5 mmol), and HBTU (0.190 g, 0.5 mmol) were dissolved in anhydrous DMF (3 mL) and DCM (1 mL). DIEA (0.087 mL, 0.5 mmol) was added and the resulting solution was added to the peptide vessel containing the resin (0.1 mmol). The vessel was allowed to rock for 3 hours at room temperature, the solution was drained, and the resin was rinsed with DMF (3 mL x 2). A solution of 1:1 DCM/DMF (3 mL x 2) was added to the resin and the peptide vessel was allowed to rock for 3 minutes. The solution was drained, and DMF (3 mL) was added. The vessel was rocked for 3 minutes, and the solvent was drained. The resin 5 (0.05 mmol) was suspended in anhydrous DCM (3 mL) in a peptide vessel. Pd(PPh3)4 (0.046 g, 0.04 mmol) and phenylsilane (0.12 mL, 1.0 mmol) were added. The vessel was allowed to rock for 2 hours, and the solution was drained under vacuum. The resin was rinsed with DCM (3 mL x 2), followed by washing the resin with DCM (3 mL x 2) by rocking for 3 minutes. Generation of the side chain primary amine to generate resin-bound peptide 6 was confirmed by orange resin coloration upon testing with TNBS. Sodium azide (39 mg, 0.6 mmol) was dissolved in water (1 mL). Toluene (1 mL) was added and the solution was cooled to 0°C. Trifluoromethanesulfonic anhydride (Tf2O, 0.05 mL, 0.3 mmol) was added dropwise to the solution. The reaction mixture was stirred at 0°C for 30 minutes, followed by warming to room temperature and was stirred for 4 hours at room temperature. A saturated sodium carbonate solution was added until the reaction mixture became basic, and the organic layer was separated. The aqueous layer was extracted with toluene (2 mL x 3) and organic layers were combined. To the combined organic layers were added triethyl amine (0.02 mL, 0.15 mmol) and methanol (2 mL). The solution was transferred to the fritted vessel containing resin charged with peptide 6 (0.1 mmol). Solid zinc chloride (2.7 mg, 0.02 mmol) was then added and the mixture was rocked for 12 hours. The peptide was rinsed with DCM/DMF (1:1, 5 mL x 3) and solvent was drained under vacuum. The conversion of amine 6 to azide 7 was confirmed by the negativeTNBS test result, with the resin exhibiting a pale gray color. CuI (0.248 g, 1.3 mmol), ascorbic acid (0.228 g, 1.3 mmol) and DIEA (0.3 mL, 1.7 mmol) were dissolved in DMF (2.5 mL) and pyridine (1.5 mL). The solution was poured into the peptide vessel containing resin charged with azide peptide 7 (0.1 mmol). The appropriate substituted alkyne (0.3 mmol) was dissolved in DMF (1 mL) and transferred to the peptide vessel. Thepeptide vessel was rocked for 12 hours at room temperature. The obtained resin-bound crude products were rinsed with DMF (5 mL x 2), DMF/DCM (5 mL x 2), and DMF (5 mL) for 3 minutes for each rinse, with solvent drained under vacuum after each rinse. The resin-bound peptide 8 (0.05 mmol) was deprotected and cleaved from the resin support per previously reported protocols.1 The Fmoc group was removed according to the general procedure for Fmoc-deprotection described above. Following Fmoc removal, a cleavage cocktail of TFA/H2O/TIPS/anisole (4.5 mL/0.25 mL/0.125 mL/0.125 mL) was added to the vessel, and the vessel was allowed to rock for 3 hours. The solution was collected, and the resin was rinsed with TFA (1 mL x 2) with the rinse added to the collected solution. Ice cold ether (10 mL) was added to a separatory funnel. The combined cleavage solution was poured into the funnel, followed by 5percent acetic acid in water (5 mL). The resulting organic layer was extracted with 5percent acetic acid in water (5 mL x 3). The aqueous layer and washes were combined and the remaining TFA and acetic acid were removed under reduced pressure. The triazole peptide product 8 was obtained by lyophilization, and for initial screening was dissolved in DMSO and used without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping