|
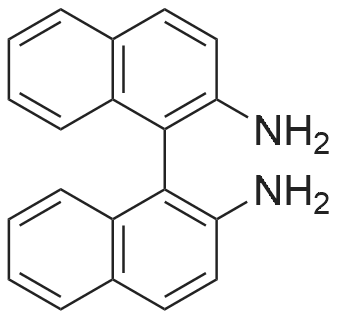
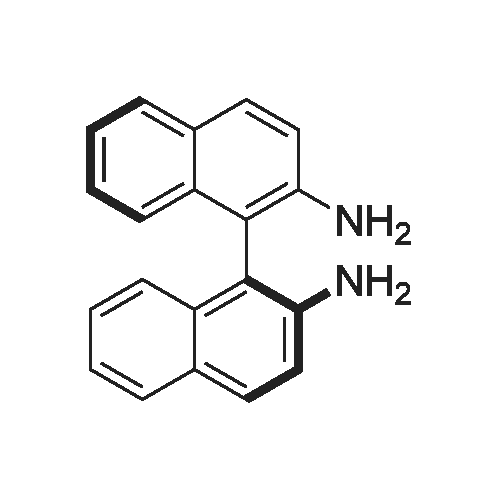
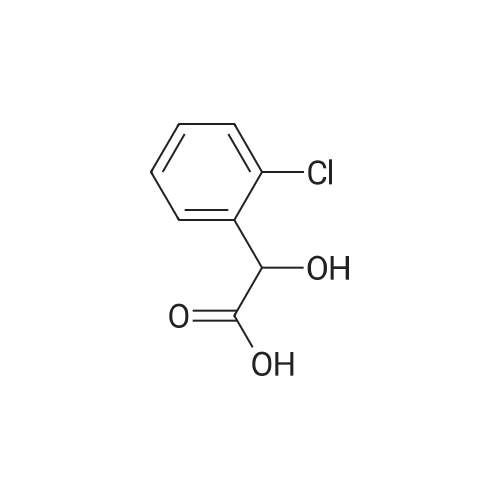
With chiral stationary phase including dimethylphenylcarbamate-functionalized CF6; In ethanol; n-heptane; at 20℃;Purification / work up; |
In addition to the foregoing, numerous other chromatographic separations using a column bonded with a CSP including a derivatized cyclofructan residue were carried out. Tables 5-9 list some additional examples of chromatographic separations using a column bonded with a CSP of the present invention. AU examples of chromatographic separations using columns bonded with CSPs of the present invention were carried out using the following experimental conditions and procedures.|0132| The high performance liquid chromatography (HPLC) column packing system was composed of an air driven fluid pump (HASKEL, DSTV- 122), an air compressor, a pressure regulator, a low pressure gauge, two high-pressure gauges (10,000 and 6,000 psi), a slurry chamber, check valves, and tubings. The CSPs were slurry packed into a 25 cm x 0.46 cm (inner diameter, I. D.) stainless steel column.|0133| The HPLC system was an Agilent 1 100 system (Agilent Technologies, Palo Alto,CA), which consisted of a diode array detector, an autosampler, a binary pump, a temperature- controlled column chamber, and Chemstation software. All chiral analytes were dissolved in ethanol, methanol, or other appropriate mobile phases, as indicated. For the LC analysis, the injection volume and flow rate were 5 muL and 1 mL/min, respectively. Separations were carried out at room temperature (~20 0C) if not specified otherwise. The wavelengths of UV detection were 195, 200, 210, and 254 nm. The mobile phase was degassed by ultrasonication under vacuum for 5 min. Each sample was analyzed in duplicate. Three operation modes (the normal phase mode, polar organic mode, and reversed phase mode) were tested, unless indicated otherwise. In the normal phase mode, heptane with ethanol or isopropanol was used as the mobile phase. In some cases, trifluoroacetic acid (TFA) was used as an additive, as indicated. The mobile phase of the polar organic mode was composed of acetonitrile/methanol and small amounts of acetic acid and triethylamine. Water/acetonitrile or acetonitrile/acetate buffer (20 mM, pH = 4.1 ) was used as the mobile phase in the reversed-phase mode.|0134| Two different supercritical fluid chromatographic instruments were used. One was a Berger SFC unit with an FCM 1200 flow control module, a TCM 2100 thermal column module, a dual pump control module, and a column selection valve. The flow rate was 4 mL/min. The cosolvent was composed of methanol/ethanol/isopropanol = 1 : 1 : 1 and 0.2percent diethylamine (DEA). The gradient mobile phase composition was 5percent cosolvent hold during 0- 0.6 min, 5-60percent during 0.6-4.3 min, 60percent hold during 4.3-6.3 min, 60percent-5percent during 6.3-6.9 min, and 5percent hold during 6.9-8.0 min. The other SFC system was a Jasco (MD, USA) system comprised of an autosampler unit (AS-2059-SF Plus), a dual pump module (PU-2086 Plus), a column thermostat module (CO-2060 Plus), a UV/Vis detector (UV-2075 Plus), and a back pressure regulator module (SCH-Vch-BP). Unless otherwise specified, the mobile phase was composed of CCVmethanol (0.1 percent TFA or 0.1percent diethylamine). The flow rate was 3 mL/min.|0135| For the calculations of chromatographic data, the "dead time" to was determined by the peak of the refractive index change due to the sample solvent or determined by injecting l ,3,5-tri-/e/-/-butylbenzene in the normal phase mode. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping