|
With ammonia; In hydrogenchloride; |
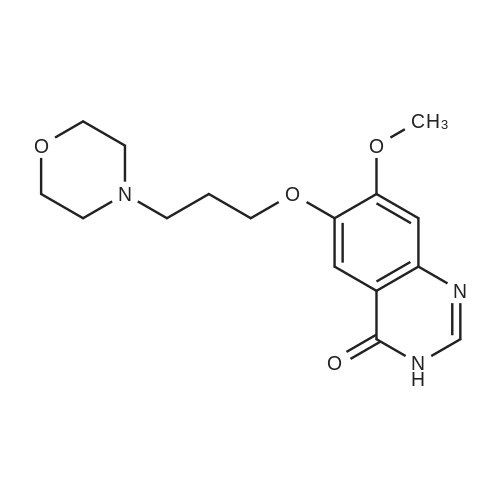
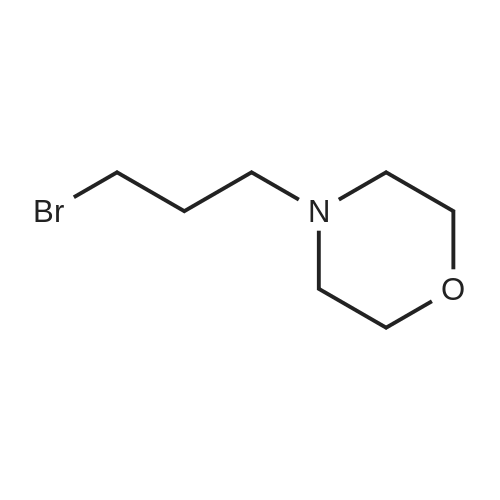
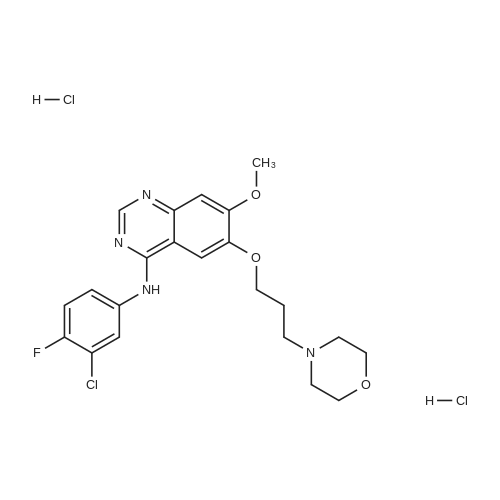
The starting material was prepared as follows: A suspension of 4-(3-chloro-4-fluoroanilino)-6-(3-morpholinopropoxy)-7-methoxyquinazoline (6.0 g 13.4 mmol), (WO 96 33980), in 6M hydrochloric acid (120 ml) was heated at reflux for 6 hours. The mixture was cooled to 0° C. and carefully neutralised with cooling by addition of concentrated aqueous ammonia. The resulting precipitate was collected by filtration, washed with dilute aqueous ammonia and water and dried under vacuum to give 7-methoxy-6-(3-morpholinopropoxy)-3,4-dihydroquinazolin-4-one (4.2 g). 1H NMR Spectrum: (DMSOd6) 2.4(m, 6H); 3.59(t, 4H); 3.75(t, 2H); 3.90(s, 3H); 4.12(t, 2H); 7.12(s, 1H); 7.43(s, 1H); 7.98(s, 1H); 12.0(br s, 1H). MS-ESI: 320 [MH]+ |
|
With ammonia; In hydrogenchloride; |
The starting material was prepared as follows: A suspension of 4-(3-chloro-4-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline (6.0 g, 13.4 mmol), (WO 96/33980), in 6M hydrochloric acid (120 ml) was heated at reflux for 6 hours. The mixture was cooled to 0° C. and carefully, with cooling, was neutralised by addition of concentrated aqueous ammonia. The resulting precipitate was collected by filtration, washed with dilute aqueous ammonia and water and dried under vacuum to give 7-methoxy-6-(3-morpholinopropoxy)-3,4-dihydroquinazolin-4-one (4.2 g, 98percentyield). 1H NMR Spectrum: (DMSOd6) 2.4(m, 6H); 3.59(t, 4H); 3.75(t, 2H); 3.90(s, 3H); 4.12(t, 2H); 7.12(s, 1H); 7.43 (s, 1H); 7.98 (s, 1H); 12.0(br s, 1H) MS-ESI: 320 [MH]+ |
|
|
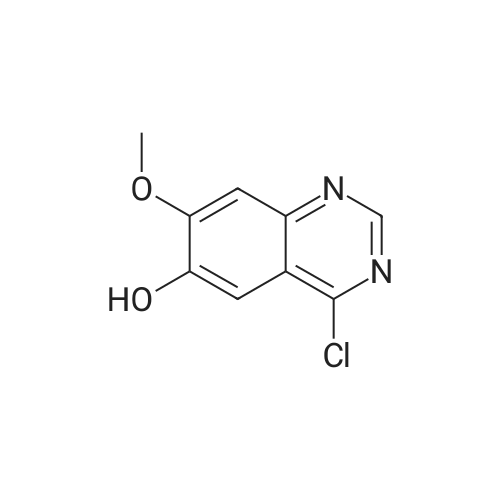
The 4-amino-7-methoxy-6-(3-morpholinopropoxy)quinazolile used as a starting material was prepared as follows :- A mixture of 4-(3-chloro-4-fluoroanhino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline (International Patent Application WO 96/33 980, Example 1 therein; 6 g) and 6N aqueous hydrochloric acid solution (120 ml) was stirred and heated to reflux for 6 hours. The mixture was cooled to 0° C. and carefully, with cooling, was neutralised by the addition of concentrated aqueous ammonium hydroxide solution. The No. resultant precipitate was isolated, washed in turn with a dilute aqueous ammonium hydroxide solution and with water and dried under vacuum. There was thus obtained 7-methoxy-6-(3-morpholinopropoxy)-3,4-dihydroquinazolin-4-one (4.2 g); NMR Spectrum: (DMSOd6) 2.4 (m, 6H), 3.59 (t, 4H), 3.75 (t, 2H), 3.9 (s, 3H), 4.12 (t, 2H), 7.12 (s, 1H), 7.43 (s, 1H), 7.98 (s, 1H), 12.0 (br s, 1H); Mass Spectrum: M + H+ 320. A mixture of a portion (0.99 g) of the material so obtained, thionyl chloride (10 ml) and DMF (0.1 ml) was stirred and heated to 80° C. for 1.5 hours. The mixture was cooled to ambient temperature, toluene (10 ml) was added and the mixture was evaporated. The residue was partitioned between ethyl acetate and water (the acidity of the aqueous layer being adjusted to pH 7.5 by the addition of 2N aqueous sodium hydroxide solution). The organic layer was washed No. with brine, dried over magnesium sulphate and evaporated. The residue was purified by column chromatography on silica using a 9:1 mixture of methylene chloride and methanol as eluent. The solid so obtained was triturated under hexane, reisolated and washed with diethyl ether. There was thus obtained 4-chloro-7-methoxy-6-(3-morpholinopropoxy)quinazoline (0.614 g); NMR Spectrum: (CDCl3) 2.12 (m, 2H), 2.5 (br s, 4H), No. 2.59 (t, 2H), 3.73 (t, 4H), 4.05 (s, 3H), 4.27 (t, 2H), 7.33 (s, 1H), 7.4 (s, 1H), 8.86 (s, 1H). A mixture of 4-chloro-7-methoxy-6-(3-morpholinopropoxy)quinazoline (1.6 g) and isopropanol (50 ml) was placed in a Carius tube which was cooled to -78° C. prior to the addition of liquid ammonia (10 ml). The Carius tube was sealed and heated to 130° C. for 20 hours. The Carius tube was cooled to ambient temperature, opened and the mixture was evaporated. The residue was triturated under diethyl ether. There was thus obtained 4- No.amino-7-methoxy-6-(3-morpholinopropoxy)quinazoline (containing 2.9 equivalents of ammoniuin chloride; 1.54 g) which was used without further purification. A portion of the material was purified by column chromatography on silica using a 19:1 mixture of methylene chloride and methanol as eluent. The purified product gave the following data :- NMR Spectrum: (DMSOd6) 1.95 (m, 2H), 2.5 (m, 6H), 3.6 (m, 4H), 3.9 (s, 3H), 4.1 (m, 2H), 7.05 (s, 1H), 7.4 (br s, 2H), No. 7.6 (s, 1H), 8.25 (s, 1H); Mass Spectrum: M + H+ 319. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping