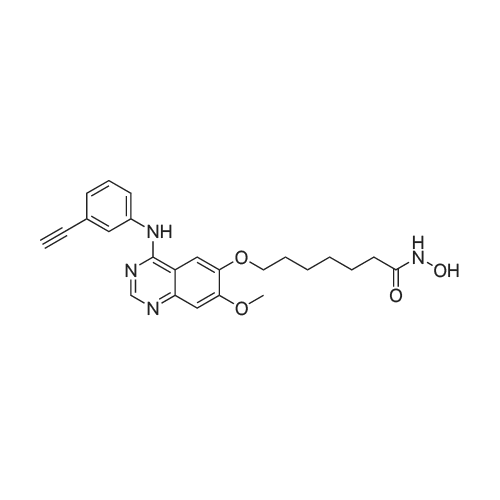
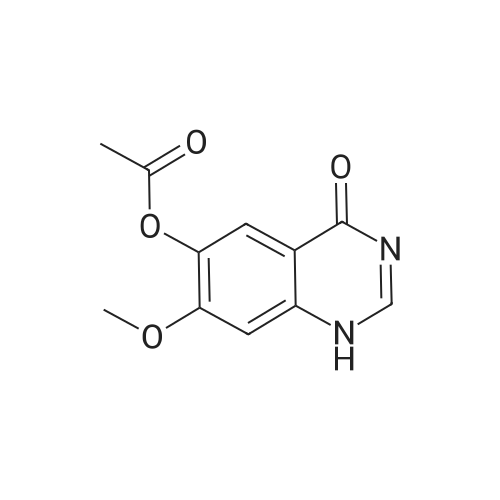
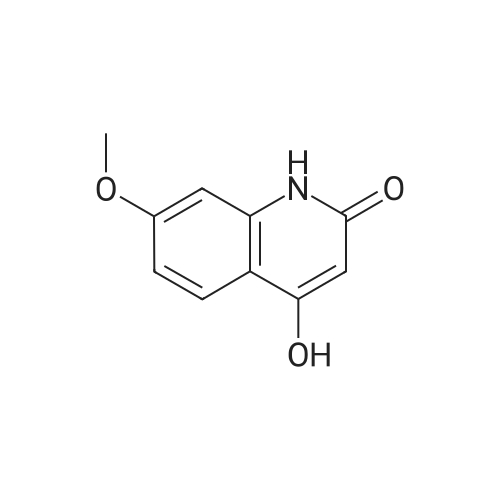
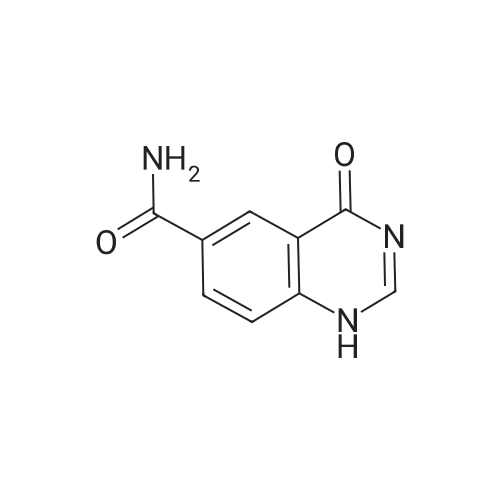
| 93 - 96% |
With pyridine; at 100℃; for 4h; |
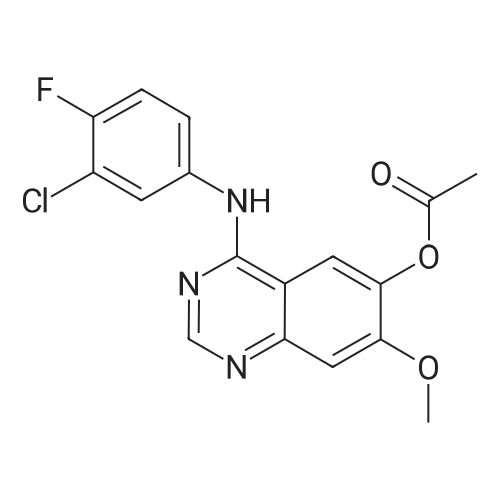
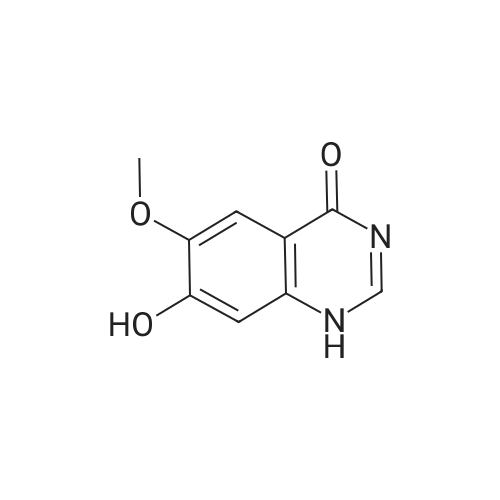
Step 5. A mixture of product step 4 (30.5 mmol), acetic anhydride (21.5 mL, 229 mmol) and pyridine (4.9 mL, 61 mmol) was heated to 100C for 4 h. After cooling to r.t., ice water (200 mL) was added and the mixture was vigorously stirred for 1 h. The precipitated 7-acetoxy-6-methoxy-3,4-dihydroquinazolin-4-one or 6-acetoxy-7-methoxy-3,4-dihydroquinazolin-4-one, respectively, was filtered off, washed with water and dried (93-96%). |
| 93 - 96% |
With pyridine; at 100℃; for 4h; |
Step 5. A mixture of product step 4 (30.5 mmol), acetic anhydride (21.5 mL, 229 mmol) and pyridine (4.9 mL, 61 mmol) was heated to 100 C. for 4 h. After cooling to r.t., ice water (200 mL) was added and the mixture was vigorously stirred for 1 h. The precipitated 7-acetoxy-6-methoxy-3,4-dihydroquinazolin-4-one or 6-acetoxy-7-methoxy-3,4-dihydroquinazolin-4-one, respectively, was filtered off, washed with water and dried (93-96%). |
| 82% |
In pyridine; at 116℃; for 3h; |
6-Acetoxy-7-methoxy-3,4-dihydroquinazolin-4-one was obtained according to W096/33980 in 82% yield. The crude product was used for the next step without purification. |
| 75% |
at 120 - 125℃; for 3h; |
Into a reaction flask, acetic anhydride (864g, 16.25 m.eq) followed by 6-hydroxy- 7-methoxy-quinazoline-4(3H)-one of formula-XII (lOOg; 1.0 m.eq) were added under stirring and heated the reaction mass to 120-125C and maintained for about 3h. The progress of the reaction was monitored by TLC. After completion of reaction, cooled the reaction mass to 25-35C and quenched in ice water (2000g) under stirring. The resulting product mixture was heated to 60-65C, maintained for lh and cooled the reaction mass to 25-35C and maintained under stirring for 2h. The resulting product was isolated by filtration and washed with water. The product was further purified by recrystallization from dimethylformamide to afford 6-acetoxy-7-methoxy-quinazoline-4(3H)-one of formula-XV as crystalline solid (90.0g; 75% by theory). |
| 60% |
With pyridine; dmap; at 100℃; for 4h; |
The 6-hydroxy-7-methoxy -3H quinazolin-4-one (4g, 0.021mol), acetic anhydride (30 ml), pyridine (5 ml) and DMAP (10 mg) is added to the 50 ml reaction flask, stirring and heating to 100 C, reaction 4h, by adding ice water, filtered, ice water washing, to obtain white powdery solid, yield 60%. |
| 53% |
With pyridine; at 20 - 100℃; for 3h; |
To a suspension of 6-hydroxy-7-methoxyquinazolin-4(3H)-one (0.57 g) and pyridine (4 mL) was added acetic anhydride ( 10 mL) at room temperature. The reaction mixture was stirred at 100 C for 3 hours, and then poured into ice-water. The resulting mixture was filtered to give the title compound (0.40 g, 53.00 %). The compound was characterized by the following spectroscopic data: ? NMR (400 MHz, d6-DMSO) ?: 2.30 (s, 3H), 3.92 (s, 3H), 7.28 (s, 1 H), 7.75 (s, 1 H), 8.08 (s, 1 H). |
| 53% |
With pyridine; at 100℃; for 3h; |
To a suspension of 6-hydroxy-7-methoxyquinazolin-4(3H)-one (0.57 g) and pyridine (4 mL) was added acetic anhydride (10 mL) at room temperature. The reaction mixture was stirred at 100 C. for 3 hours, and then poured into ice-water. The resulting mixture was filtered to give the title compound (0.40 g, 53.00%). The compound was characterized by the following spectroscopic data: 1H NMR (400 MHz, d6-DMSO) δ: 2.30 (s, 3H), 3.92 (s, 3H), 7.28 (s, 1H), 7.75 (s, 1H), 8.08 (s, 1H). |
| 51% |
With pyridine; at 100℃; for 6h; |
Add 6-hydroxy-7-methoxy-3H-quinazolin-4-one (1 g, 5.2 mmol) in a 50 mL round bottom flask, acetic anhydride(20mL) and pyridine (4mL), the system was reacted at 100 C for 6h,The TLC detection of the starting material was complete.The reaction system was cooled to room temperature, and the round bottom flask was placed in an ice water bath and stirred.The suspension was then transferred to a 500 mL round bottom flask.In an ice water bath, add 300 mL of ice water and stir for 20 minutes.A large amount of white solid precipitated. Filter by suction, then wash the filter cake twice with water.Drying in an oven, the obtained white solid is (7-methoxy-4-oxo-3,4-dihydroquinazoline)-6-yl-acetate, yield51%. |
| 50% |
With pyridine; for 3h;Heating / reflux; |
A mixture of 6-hydroxy-7-methoxyquinazolin-4(3H)-one (0103) (1O g crude), acetic anhydride (100 ml) and pyridine (8ml) was stirred and heated to reflux for 3 hours. The mixture was cooled to room temperature and poured into a mixture (250ml) of ice and water. The precipitate was isolated and dried to yield the title product 0104 as a grey solid (5.8g, 50% two step overall yield): LCMS: m/z 235[M+1]+; 1H NMR(CDCl31) δ 2.27 (s, 3H), 3.89 (s, 3H), 7.28 (s, IH), 7.72 (s, IH), 8.08 (d, IH), 12.20 (bs, IH). |
| 40% |
With pyridine; dmap; at 100℃; for 6h;Inert atmosphere; |
A suspension of compound 3 (3.88 g, 20.2 mmol), pyridine (4 mL) and DMAP (122 mg, 1.0 mmol) in acetic anhydride (30 mL) was heated to 100 C and stirred under N2 atmosphere for 6 h. The reaction mixture was cooled and poured into ice-water. The resultant precipitate was filtered, washed with water and dried under vacuum to afford 4 (1.88 g, 40%) as a beige solid, mp 303-305 C. 1H NMR (DMSO-d6): d 12.2 (s, 1H), 8.09 (s, 1H), 7.75 (s, 1H), 7.28 (s, 1H), 3.91 (s,3H), 2.30 (s, 3H). LC-MS (ESI, m/z): calcd for C11H11N2O4 ([M+H]+) 235.1, found 235.1. |
|
In pyridine; for 3h;Heating / reflux; |
A mixture of 6-hydroxy-7-methoxyquinazolin-4(3H)-one (0103) (1O g crude), acetic anhydride (100 ml) and pyridine (8ml) was stirred and heated to reflux for 3 hours. The mixture was cooled to room temperature and poured into a mixture (250ml) of ice and water. The precipitate was isolated and dried to yield the title product 0104 as a grey solid (5.8g, 50% two step overall yield): LCMS: m/z 235[M+1]+; 1H NMR(CDCU) δ 2.27 (s, 3H), 3.89 (s, 3H), 7.28 (s, IH), 7.72 (s, IH), 8.08 (d, IH), 12.20 (bs, IH). |
|
With pyridine; for 3h;Heating / reflux; |
Step 1c. 3,4-Dihydro-7-methoxy-4-oxoquinazolin-6-yl acetate (Compound 0104) A mixture of 6-hydroxy-7-methoxyquinazolin-4(3H)-one (0103) (10 g crude), acetic anhydride (100 ml) and pyridine (8 ml) was stirred and heated to reflux for 3 hours. The mixture was cooled to room temperature and poured into a mixture (250 ml) of ice and water. The precipitate was isolated and dried to yield the title product 0104 as a grey solid (5.8 g, 50% two step overall yield): LCMS: m/z 235 [M+1]+; 1H NMR (CDCl3.) δ 2.27 (s, 3H), 3.89 (s, 3H), 7.28 (s, 1H), 7.72 (s, 1H), 8.08 (d, 1H), 12.20 (bs, 1H). |
|
With pyridine; at 20℃; |
The compound of 15g GG3 placed 40ml of pyridine was added acetic anhydride (1.5 eq), stirred at room temperature overnight, the reaction was complete, poured into water, the precipitated solid was filtered to give compound GG4. |
|
With pyridine; for 3h;Heating / reflux; |
Step 1c. 3,4-Dihydro-7-methoxy-4-oxoquinazolin-6-yl Acetate (Compound 0104); A mixture of 6-hydroxy-7-methoxyquinazolin-4(3H)-one (0103) (10 g crude), acetic anhydride (100 ml) and pyridine (8 ml) was stirred and heated to reflux for 3 hours. The mixture was cooled to room temperature and poured into a mixture (250 ml) of ice and water. The precipitate was isolated and dried to yield the title product 0104 as a grey solid (5.8 g, 50% two step overall yield): LCMS: m/z 235[M+1]+; 1H NMR (CDCl3) δ 2.27 (s, 3H), 3.89 (s, 3H), 7.28 (s, 1H), 7.72 (s, 1H), 8.08 (d, 1H), 12.20 (bs, 1H). |
|
|
6-Hydroxy-7-methoxy-4-ketoquinazoline (20 g, 0.11 mol, ie compound B1), acetic anhydride (150 mL, 1.6 mol) and pyridine (20 mL, 0.25 mol) were sequentially added to a 500 mL circle In the bottom flask, after heating to 100 C for 1 h, add 4-dimethylaminopyridine (0.9 g, 0.0073πο 1), continue the reaction for 5 h, stop the reaction, evaporate acetic anhydride under reduced pressure, and add cold to the reaction solution. The saturated sodium carbonate solution (500 mL) was stirred, suction filtered, and the filter cake was transferred to a round bottom flask and stirred with a large amount of ice water, suction filtered, and the filter cake was washed with distilled water until ΡΗ = 7, and dried to give a yellow-white solid ( Compound B2). This was sequentially added to a 500 mL three-neck round bottom flask with thionyl chloride (190 mL, 2.0 mol), and the mixture was heated to 80 C for reflux for 20 min, and N,N-dimethylformamide was slowly added from a constant pressure dropping funnel. (4.4 mL, 0.52 eq) was added dropwise to the reaction mixture. After reacting for 6 h, the reaction was stopped, cooled, and the thionyl chloride was recovered under reduced pressure. Toluene was added with stirring, and toluene was added under reduced pressure. Toluene (250 mL) was added to the reaction mixture and stirred for about 1 h, dichloromethane (120 mL*2) Extract and separate the organic layer,Wash with water (80 mL * 2), dry over anhydrous sodium sulfate, and dilute dichloromethane under reduced pressure.Obtained 21.5 g of an off-white solid, yield: 81.9%. |
| 117 g |
With pyridine; In N,N-dimethyl-formamide; at 40 - 45℃; for 1h; |
100 g (0.52 mol) of the compound of the formula VI, 500 mL of N,N-dimethylformamide and 200 mL of pyridine were added to a 2 L three-necked flask, and 500 mL of acetic anhydride was added dropwise thereto, and the mixture was added dropwise, and the mixture was heated to 40-45 C for 1 hour. The end of the reaction was monitored by HPLC. The reaction solution was poured into 1.2 L of ice water, crystallized, filtered, and the solid was collected, and dried at 50 C to obtain a light brown compound I 117 g, a molar yield of 96%, a purity of 99%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping