| 152 mg |
|
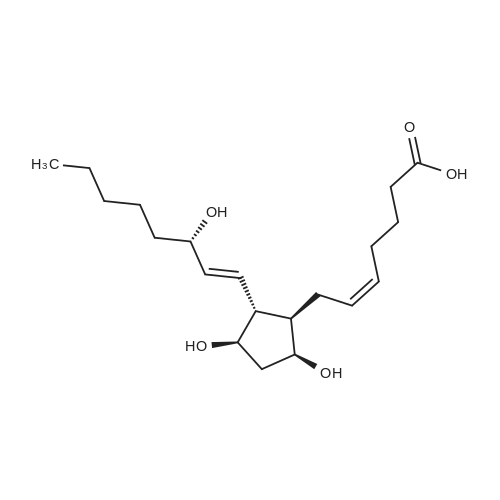
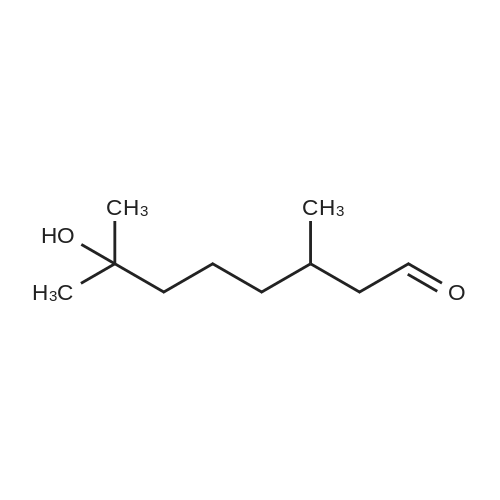
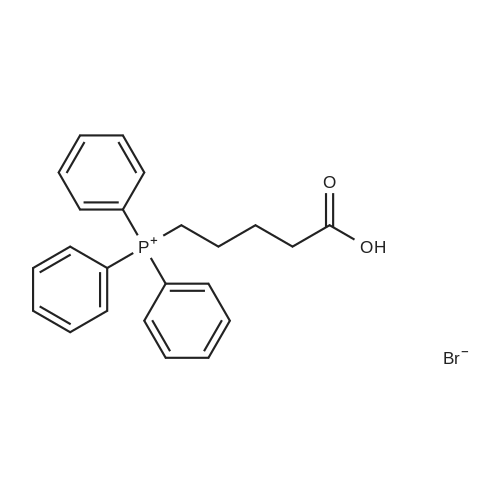
4-Carboxybutyl)(triphenyl)phosphonium bromide 29 (de los Angeles Rey, M. et al., J. Org. Chem. 64, 3196-3206 (1999) Note: Material prepared as described in this reference, but using toluene in place of benzene and pentane in place of hexane during the washing of the product) (2.00 g, 4.52 mmol), which corresponds to compound (VIII) described above, was added to a flame dried schlenk flask, under N2, and anhydrous THF (16.0 ml) added. The resulting suspension was cooled to 0 C. KOt-Bu (1.01 g, 9.03 mmol) was added in one portion and the resulting orange mixture stirred at 0 C for 40 min. A solution of crude triol 28 (203 mg, 0.75 mmol) in anhydrous THF (4.0 ml) was added dropwise via syringe. After complete addition the mixture was stirred at r.t. for 1 h. The reaction was quenched with H20 (25 ml) and washed with Et20 (2 x 25 ml) to remove triphenylphosphine oxide. The aqueous phase was made acidic with 1 M HCI (~10 ml) and extracted with CH2CI2 (5 x 25 ml). The combined organic phases were dried (MgS04), filtered, and concentrated to give the crude material. This was triturated with EtOAc/heptane and the solids filtered and washed with EtOAc (4 x 5 ml). The filtrate was concentrated under vacuum and purified by column chromatography on silica, eluting with EtOAc/petrol/AcOH (60:35:5) to give PGF2a. This was dissolved in CH2CI2 and washed with H20 (5 ml). The organic phase was then dried (MgS04), filtered, and concentrated to give PGF2a (152 mg, 57% over 2 steps) as a clear, colourless oil. The H NMR data was consistent with that reported by Mulzer (Sheddan, N. A. et al., Org. Lett. 8, 3101-3104 (2006)). The 13C NMR data was in excellent agreement with that reported by Parve (Parve, O. et al., Bioorg. Med. Chem. Lett. 9, 1853-1858 (1999)). The IR and optical rotation data are in agreement with that reported by Mulzer (Sheddan, N. A. et al., Org. Lett. 8, 3101-3104 (2006)) and Corey (Corey, E. et ai, J. Am. Chem. Soc. 92, 397-398 (1970)). Rf = 0.24 (EtOAc:40/60 petroleum ether:AcOH, 60:35:5) vmax (neatVcm-1 3339, 2961, 2930, 2857, 2490, 1705, 1457, 1380, 1245, 1118, 1086, 1047, 970, 910, 878, 731 *H NMR (400 MHz; CDCfe) deltaEta = 0.89 (3 H, t, J = 6.8 Hz, CH3), 1.24-1.41 (6 H, m, 3 x CH2), 1.43-1.54 (2 H, m,), 1.54-1.63 (1 H, m), 1.63-1.73 (2 H, m), 1.76 (1 H, m), 2.07-2.28 (5 H, m), 2.28-2.39 (3 H, m), 3.96 (1 H, m, CAOH), 4.11 (1 H, q, J = 6.8 Hz, CAOH), 4.18 (1 H, m, CAOH), 4.35-5.20 (1 H, br. s, C02H), 5.32-5.41 (1 H, m, =CH), 5.41-5.50 (1 H, m, =CH), 5.50 (1 H, dd, J = 15.4, 8.4 Hz, =CH), 5.58 (1 H, dd, J = 15.4, 6.6 Hz, =CH) 13C NMR (125 MHz; CDCI3) 5C = 14.0 (CH3), 22.6 (CH2), 24.5 (CH2), 25.2 (CH2), 25.2 (CH2), 26.3 (CH2), 31.7 (CH2), 33.1 (CH2), 36.9 (CH2), 42.7 (CH2), 50.0 (CH), 55.2 (CH), 72.3 (ACOH), 73.2 (ACOH), 77.4 (ACOH), 129.2 (=CH), 129.5 (=CH), 132.8 (=CH), 135.1 (=CH), 177.5 (C=0) HRMS (ESI) calcd for Q^OsNa [MNa+] 377.2298, found 377.2303 [a]D22 -23.5 (c. 1.0, THF) (lit.,49 [a]D20 -24.9 (c. 0.57, THF)) (lit.,51 [a]D25 -23.8 (synthetic material) (c. 1.0, THF)) (lit.,51 [a]D25 -23.5 (natural material) (c. 1.0, THF)) |
| 152 mg |
|
5F. (Z)-7-(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(E,3S)-3-hydroxy-1-octenyl]cyclopentyl-5-heptenoic acid, PGF2alpha (1) (4-Carboxybutyl)(triphenyl)phosphonium bromide 29 (de los Angeles Rey, M. et al., J. Org. Chem. 64, 3196-3206 (1999) Note: Material prepared as described in this reference, but using toluene in place of benzene and pentane in place of hexane during the washing of the product) (2.00 g, 4.52 mmol), which corresponds to compound (VIII) described above, was added to a flame dried schlenk flask, under N2, and anhydrous THF (16.0 ml) added. The resulting suspension was cooled to 0 C. KOt-Bu (1.01 g, 9.03 mmol) was added in one portion and the resulting orange mixture stirred at 0 C. for 40 min. A solution of crude triol 28 (203 mg, 0.75 mmol) in anhydrous THF (4.0 ml) was added dropwise via syringe. After complete addition the mixture was stirred at r.t. for 1 h. The reaction was quenched with H2O (25 ml) and washed with Et2O (2*25 ml) to remove triphenylphosphine oxide. The aqueous phase was made acidic with 1 M HCl (?10 ml) and extracted with CH2Cl2 (5*25 ml). The combined organic phases were dried (MgSO4), filtered, and concentrated to give the crude material. This was triturated with EtOAc/heptane and the solids filtered and washed with EtOAc (4*5 ml). The filtrate was concentrated under vacuum and purified by column chromatography on silica, eluting with EtOAc/petrol/AcOH (60:35:5) to give PGF2alpha. This was dissolved in CH2Cl2 and washed with H2O (5 ml). The organic phase was then dried (MgSO4), filtered, and concentrated to give PGF2alpha (152 mg, 57% over 2 steps) as a clear, colourless oil. The 1H NMR data was consistent with that reported by Mulzer (Sheddan, N. A. et al., Org. Lett. 8, 3101-3104 (2006)). The 13C NMR data was in excellent agreement with that reported by Parve (Parve, O. et al., Bioorg. Med. Chem. Lett. 9, 1853-1858 (1999)). The IR and optical rotation data are in agreement with that reported by Mulzer (Sheddan, N. A. et al., Org. Lett. 8, 3101-3104 (2006)) and Corey (Corey, E. et al., J. Am. Chem. Soc. 92, 397-398 (1970)). Rf=0.24 (EtOAc:40/60 petroleum ether:AcOH, 60:35:5) numax (neat)/cm-1 3339, 2961, 2930, 2857, 2490, 1705, 1457, 1380, 1245, 1118, 1086, 1047, 970, 910, 878, 731 1H NMR (400 MHz; CDCl3) deltaH=0.89 (3H, t, J=6.8 Hz, CH3), 1.24-1.41 (6H, m, 3*CH2), 1.43-1.54 (2H, m,), 1.54-1.63 (1H, m), 1.63-1.73 (2H, m), 1.76 (1H, m), 2.07-2.28 (5H, m), 2.28-2.39 (3H, m), 3.96 (1H, m, CHOH), 4.11 (1H, q, J=6.8 Hz, CHOH), 4.18 (1H, m, CHOH), 4.35-5.20 (1H, br. s, CO2H), 5.32-5.41 (1H, m, =CH), 5.41-5.50 (1H, m, =CH), 5.50 (1H, dd, J=15.4, 8.4 Hz, =CH), 5.58 (1H, dd, J=15.4, 6.6 Hz, =CH) 13C NMR (125 MHz; CDCl3) deltaC=14.0 (CH3), 22.6 (CH2), 24.5 (CH2), 25.2 (CH2), 25.2 (CH2), 26.3 (CH2), 31.7 (CH2), 33.1 (CH2), 36.9 (CH2), 42.7 (CH2), 50.0 (CH), 55.2 (CH), 72.3 (HCOH), 73.2 (HCOH), 77.4 (HCOH), 129.2 (=CH), 129.5 (=CH), 132.8 (=CH), 135.1 (=CH), 177.5 (C=O) HRMS (ESI) calcd for C20H34O5Na [MNa+] 377.2298. found 377.2303. [alpha]D22 -23.5 (c. 1.0, THF) (lit., 49[alpha]D20 -24.9 (c. 0.57, THF)) (lit., 51[alpha]D25 -23.8 (synthetic material) (c. 1.0, THF)) (lit., 51[alpha]D25 -23.5 (natural material) (c. 1.0, THF)) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping