| 87% |
|
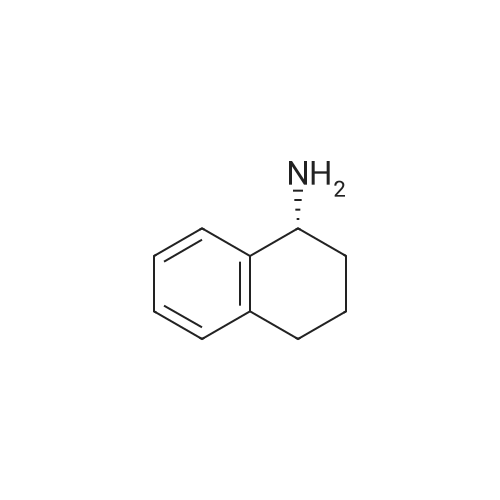
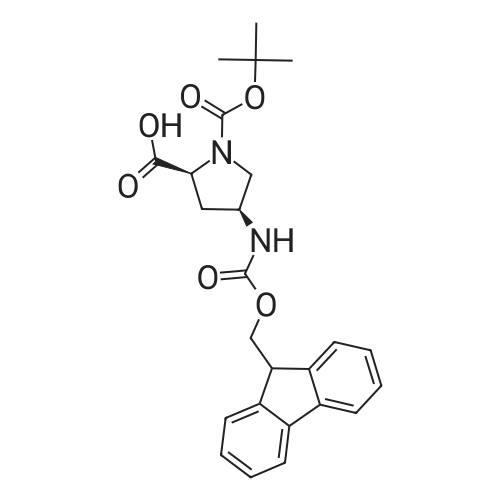
A) (2S,4S)-tert-Butyl 4-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-2-(((R)- 1 ,2,3 ,4-tetrahydronaphthalen- 1 -yl)carbamoyl)pyrrolidine- 1 -carboxylate [00147] To a solution of (2S,4S)-Boc-gamma-(Fmoc-amino)-proline (Chem-Impex, 6.00 g, 13.3 mmol) in DMF (20 mL) at 0 C were added EDC (3.05 g, 15.9 mmol), HOAt (2.17 g, 15.9 mmol) and 4-methylmorpholine (4.38 mL, 39.8 mmol). The reaction mixture was stirred at ice bath temperature for 20 min then treated with a solution of (R)- 1,2,3,4-tetrahydronaphthalen-l -amine (ALFA AESAR, 2.15 g, 14.6 mmol) in DMF (2 mL). The reaction mixture was stirred at rt for 1 h and cold water (100 mL) was added to the reaction mixture. The solid that formed was collected by filtration and washed with cold water (100 mL). The solid was dissolved in CH2CI2 (200 mL) and the organic solution was washed with 5% aq. citric acid solution and brine, dried over MgS04, and filtered. The filtrate was concentrated in vacuo. The residue was dissolved in CH2CI2 and purified by flash column chromatography (gradient elution from 10 to 30% EtOAc in CH2CI2) provided the title compound (6.7 g, 87%) as a light tan solid. XH NMR (400 MHz, CDCI3) δ 7.79 (d, J= 7.5 Hz, 2H), 7.67 (d, J= 7.3 Hz, 2H), 7.42 (td, J= 7.2, 4.0 Hz, 2H), 7.37 - 7.03 (m, 6H), 5.22 (br. s., 1H), 4.57 - 4.23 (m, 5H), 3.68 - 3.49 (m, 2H), 2.91 - 2.74 (m, 2H), 2.52 (d, J= 13.4 Hz, 1H), 2.35 - 2.21 (m, 1H), 2.14 (d, J= 5.1 Hz, 1H), 1.97 - 1.80 (m, 3H), 1.44 (s, 9H); MS(ESI+) m/z 582.2 (M+H)+. |
| 87% |
|
To a solution of (2S,4S)-Boc-4-(Fmoc-amino)-proline (Chem-Impex, 6.00 g, 13.3 mmol) in DMF (20 mL) at 0 C. were added EDC (3.05 g, 15.9 mmol), HOAt (2.17 g, 15.9 mmol) and NMM (4.38 mL, 39.8 mmol). The reaction mixture was stirred at ice bath temperature for 20 min then treated with a solution of (R)-1,2,3,4-tetrahydronaphthalen-1-amine (ALFA AESAR, 2.15 g, 14.6 mmol) in DMF (2 mL). The reaction mixture was stirred at rt for 1 h and cold water (100 mL) was added to the reaction mixture. The solid that formed was collected by filtration and washed with cold water (100 mL). The solid was dissolved in CH2Cl2 (200 mL) and the organic solution was washed with 5% aq. citric acid solution and brine, dried over MgSO4, and filtered. The filtrate was concentrated in vacuo. The residue was dissolved in CH2Cl2 and purified by flash column chromatography (gradient elution from 10 to 30% EtOAc in CH2Cl2) provided the title compound (6.70 g, 87%) as a light tan solid. 1H NMR (400 MHz, CDCl3) δ 7.79 (d, J=7.5 Hz, 2H), 7.67 (d, J=7.3 Hz, 2H), 7.42 (td, J=7.2, 4.0 Hz, 2H), 7.37-7.03 (m, 6H), 5.22 (br. s., 1H), 4.57-4.23 (m, 5H), 3.68-3.49 (m, 2H), 2.91-2.74 (m, 2H), 2.52 (d, J=13.4 Hz, 1H), 2.35-2.21 (m, 1H), 2.14 (d, J=5.1 Hz, 1H), 1.97-1.80 (m, 3H), 1.44 (s, 9H); MS(ESI+) m/z 582.2 (M+H)+. |
| 87% |
|
To a solution of (2S,4S)-l3oc-gamma-(Fmoc- amino)-proline (Chem-Impex, 6.00 g, 13.3 mmol) in DMF (20 mE) at 00 C. were added EDC (3.05 g, 15.9 mmol), HOAt (2.17 g, 15.9 mmol) and NMM (4.38 mE, 39.8 mmol). The reaction mixture was stirred at ice bath temperature for 20 mm then treated with a solution of (R)-1 ,2, 3,4-tetrahydronaphthalen- i-amine (Alfa Aesar, 2.15 g, 14.6 mmol) in DMF (2 mE). The reaction mixture was stirred at it for 1 hand cold water (100 mE) was added to the reaction mixture. The solid that formed was collected by filtration and washed with cold water (100 mE). The solid was dissolved in CH2C12 (200 mE) and the organic solution was washed with 5% aq. citric acid solution and brine, dried over MgSO4, and filtered. The filtrate was concentrated in vacuo. The residue was dissolved in CH2C12 and purified by flash colunm chromatography (gradient elution from 10 to 30% EtOAc in CH2C12) provided the title compound (6.70 g, 87%) as a light tan solid. ‘H NMR (400 MHz, CDC13) ? 7.79 (d, J=7.5 Hz, 2H), 7.67 (d, J7.3 Hz, 2H), 7.42 (td, J=7.2, 4.0 Hz, 2H), 7.37-7.03 (m, 6H), 5.22 (bt s., 1H), 4.57-4.23 (m, 5H), 3.68-3.49 (m, 2H), 2.91-2.74 (m, 2H), 2.52 (d, J=13.4 Hz, 1H), 2.35-2.21 (m, 1H), 2.14 (d, J=5.i Hz, 1H), 1.97- 1.80 (m, 3H), 1.44 (s, 9H); MS(ESI) mlz 582.2 (M+H). |
| 9.6 g |
With N-ethyl-N,N-diisopropylamine; HATU; In N,N-dimethyl-formamide; at 0℃; for 4h; |
To a solution of (2S,4S )-4-(9H-fluoren-9 -ylmethoxycarbonylamino)-pyrrolidine- 1,2-dicarboxylic acid 1-tert-butyl ester (Aldrich) (7.5 g, 16.593 mmol) in DMF (100 mL) were addedHATU (6.93 g, 18.252 mmol) and DIPEA (14.36 mL, 82.965 mmol) and the mixture was cooled to 0 C. (R)-(1,2,3,4-tetrahydro-naphthalen-1-yl)amine (2.44 g, 16.593 mmol) was added dropwise and the cooling bath removed. After 4 h the mixture was diluted with ethyl acetate, washed with water, dried over sodium sulfate and concentrated to afford the title compound as an off white solid (9.6 g) which was used without purification. LC-MS: 582 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping