| 754 g |
With polyphosphoric acid; In tetrahydrofuran; diethyl ether; at -10 - 75℃; for 20h;Inert atmosphere; |
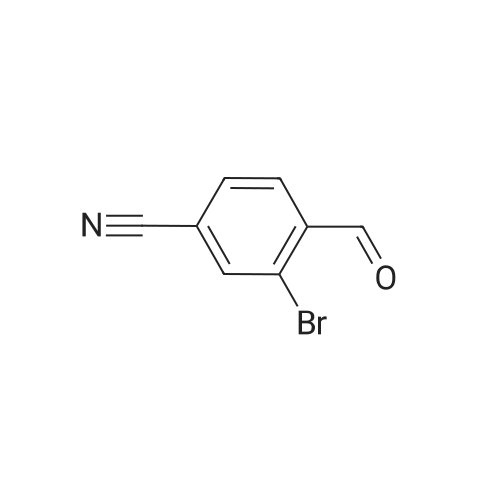
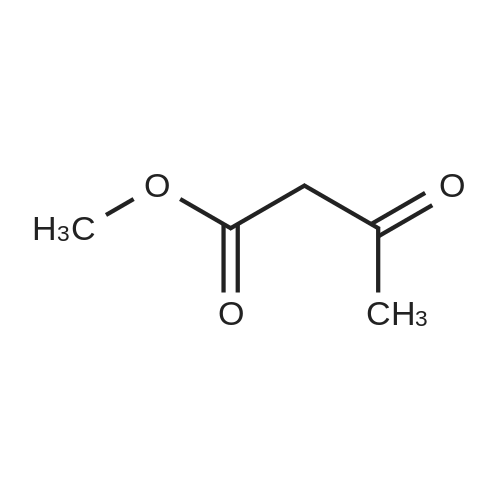
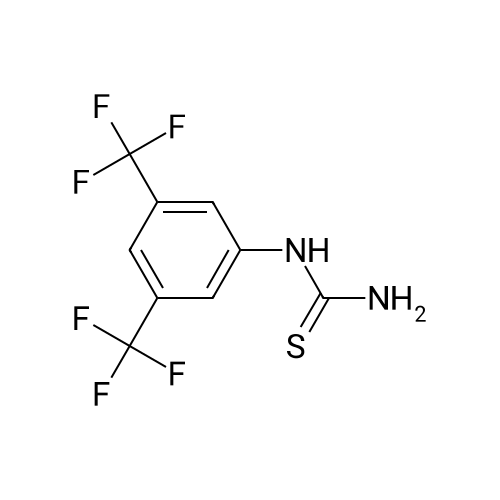
Intermediate 34 (458 g, 2 mol, 1.0 eq.), methyl acetoacetate (274.4 g, 255 mL, 2.36 mol, 1.18 eq.) and 3-trifluoromethylphenyl thiourea (519 g, 2.36 mol, 1.18 eq.), were charged to a 10 L jacketed reactor under a N2 atmosphere, and suspended in THF (4.6 L) and while stirring, was cooled to -10 C. (internal temperature -3 C.). Polyphosphoric acid (1650 g, 3.6 wt eq.), was prewarmed in a water bath at 50 C., then added in one portion, resulting in an immediate exotherm, and the internal temperature rose to 19 C. The resulting orange mixture was then warmed to 75 C. in 10 C. increments to a gentle reflux, and the reaction stirred at this temperature for 20 hours. The reaction was then cooled to 20 C. and the bulk of THF removed in vacuo to give a dark orange viscous oil, which was then diluted with water (5 L) and Et2O (5 L).The aqueous layer was separated and extracted again with Et2O (2*2 L) and the combined organics were subsequently washed with water (1 L), brine (1 L) and dried (Na2SO4) and filtered through Celite to remove any fine particulates. The filtered solution was then concentrated in vacuo to give a viscous orange gum which was resuspended in Et2O (ca. 1.5 L) and left to stand overnight. The resulting suspension was filtered and the solid collected was rinsed with Et2O (0.5 L) and dried in a vacuum oven at 50 C. (8 mbar) for 4 days to afford the title compound (754 g). LCMS (Method 1): Rt 3.52 min, m/z 529, 531 [M+H]+ 1H NMR (300 MHz, DMSO): delta 10.15 (1H, d, J=3.5 Hz), 8.11 (1H, d, J=1.6 Hz), 8.05 (1H, dd, J=8.1, 1.7 Hz), 7.92-7.64 (5H, m), 5.80 (1H, d, J=2.9 Hz), 3.53 (3H, s), 2.07 (3H, s). |
| 754 g |
With polyphosphoric acid; In tetrahydrofuran; at -10 - 75℃; for 20h;Inert atmosphere; |
Intermediate 34 (458 g, 2 mol, 1.0 eq.), methyl acetoacetate (274.4 g, 255 mL, 2.36 mol, 1.18 eq.) and 3-trifluoromethylphenyl thiourea (519 g, 2.36 mol, 1.18 eq.), were charged to a 10 L jacketed reactor under a N2 atmosphere, and suspended in THF (4.6 L) and while stirring, was cooled to -10C (internal temperature -3C). Polyphosphoric acid (1650 g, 3.6 wt eq.), was prewarmed in a water bath at 50C, then added in one portion, resulting in an immediate exotherm, and the internal temperature rose to 19C. The resulting orange mixture was then warmed to 75C in 10C increments to a gentle reflux, and the reaction stirred at this temperature for 20 h. The reaction was then cooled to 20C and the bulk of THF removed in vacuo to give a dark orange viscous oil, which was then diluted with water (5 L) and Et20 (5 L). The aqueous layer was separated and extracted again with Et20 (2 x 2 L) and the combined organics were subsequently washed with water (1 L), brine (1 L) and dried (Na2S04) and filtered through Celite to remove any fine particulates. The filtered solution was then concentrated in vacuo to give a viscous orange gum which was resuspended in Et20 (ca. 1.5 L) and left to stand overnight. The resulting suspension was filtered and the solid collected was rinsed with Et20 (0.5 L) and dried in a vacuum oven at 50C (8 mbar) for 4 days to afford the title compound (754 g). LCMS (Method l):Rt 3.52 min, m/z 529, 531 [M+H]+ 1H NMR (300 MHz, DMSO): delta 10.15 (1H, d, J = 3.5 Hz), 8.11 (1H, d, J = 1.6 Hz), 8.05 (1H, dd, J = 8.1, 1.7 Hz), 7.92-7.64 (5H, m), 5.80 (1H, d, J = 2.9 Hz), 3.53 (3H, s), 2.07 (3H, s). |
| 754 g |
With Polyphosphoric acid; In tetrahydrofuran; at -10 - 75℃; for 20h;Inert atmosphere; Industrial scale; |
Intermediate 2 (458 g, 2 mol, 1.0 eq.), methyl acetoacetate (274.4 g, 255 mL, 2.36 mol, 1.18 eq.) and 3-trifluoromethylphenyl thiourea (519 g, 2.36 mol, 1.18 eq.), were charged to a 10 L jacketed reactor under a N2 atmosphere, and suspended in THF (4.6 L) and while stirring, was cooled to -10 C. (internal temperature -3 C.). Polyphosphoric acid (1650 g, 3.6 wt eq.), was prewarmed in a water bath at 50 C., then added in one portion, resulting in an immediate exotherm, and the internal temperature rose to 19 C. The resulting orange mixture was then warmed to 75 C. in 10 C. increments to a gentle reflux, and the reaction stirred at this temperature for 20 h. The reaction was then cooled to 20 C. and the bulk of THF removed in vacuo to give a dark orange viscous oil, which was then diluted with water (5 L) and Et2O (5 L). The aqueous layer was separated and extracted again with Et2O (2×2 L) and the combined organics were subsequently washed with water (1 L), brine (1 L) and dried (Na2SO4) and filtered through Celite to remove any fine particulates. The filtered solution was then concentrated in vacuo to give a viscous orange gum which was resuspended in Et2O (ca. 1.5 L) and left to stand overnight. The resulting suspension was filtered and the solid collected was rinsed with Et2O (0.5 L) and dried in a vacuum oven at 50 C. (8 mbar) for 4 days to afford the title compound (754 g). LCMS (Method 1): Rt 3.52 min, m/z 529, 531 [M+H]+ 1H NMR (300 MHz, DMSO): delta 10.15 (1H, d, J=3.5 Hz), 8.11 (1H, d, J=1.6 Hz), 8.05 (1H, dd, J=8.1, 1.7 Hz), 7.92-7.64 (5H, m), 5.80 (1H, d, J=2.9 Hz), 3.53 (3H, s), 2.07 (3H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping