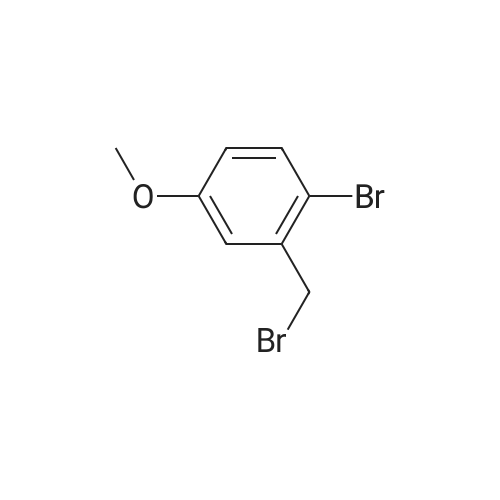
| 64% |
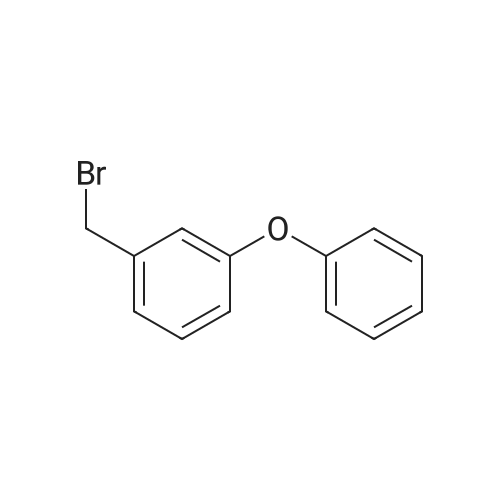
With carbon tetrabromide; triphenylphosphine; In dichloromethane; at 20℃; for 2h; |
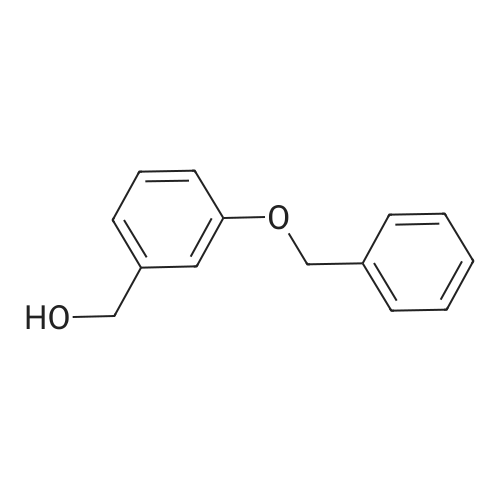
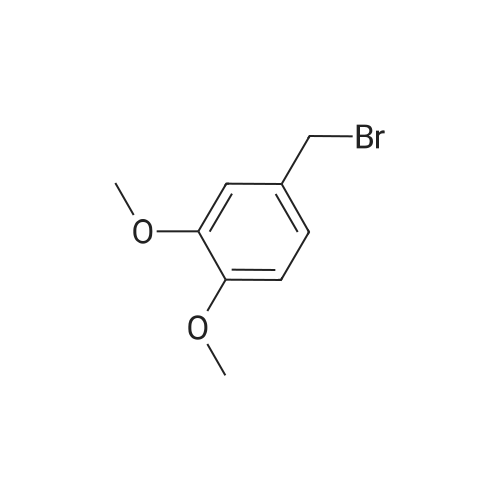
3-Hydroxybenzaldehyde (5.00 g, 0.041 mol) was dissolved in anhydrous acetonitrile (130 mL) under argon atmosphere. Cesium carbonate (20.01 g, 0.061 mol) was added and the suspension stirred for 5 min. Benzyl bromide (11.69 mL, 0.102 mol) was then added and the solution heated at reflux for 16 h. The solution was concentrated on rotary evaporator, water was added and the mixture was extracted with EtOAc. The organic phase was washed twice with water, once with brine, dried over MgSO4, filtered and concentrated. Water was added and the mixture was extracted with CH2Cl2. The crude product was purified by flash chromatography on silica gel with hexanes/EtOAc (80/20) to yield 3a as a white solid (8.59 g). 1H NMR delta (CDCl3) 5.13 (s, 2H, PhCH2O), 5.33 (s, 2H, COOCH2Ph), 7.35-7.50 (m, 9H, 2-CH, 4-CH, 5-CH, 6-CH and PhCH2O), 10.00 (s, 1H, PhCHO). The aldehyde 3a was dissolved in anhydrous THF (200 mL) under argon and cooled to 0 °C. Lithium aluminum hydride (1.55 g, 0.041 mol) was added in small portions and the solution stirred at room temperature for 2 h. The reaction was then quenched using water (0.8 mL), a 10percent wt aqueous NaOH solution (1.15 mL) and water again (1.9 mL) and left to settle. The suspension was then filtered and concentrated. Water was added and the mixture extracted with EtOAc, the organic phase dried over MgSO4, filtered and concentrated to yield 7.07 g crude alcohol 3b. 1H NMR delta (CDCl3) 4.68 (s, 2H, PhCH2OH), 5.08 (s, 2H, PhCH2O), 6.90-7.46 (m, 9H, 2-CH, 4-CH, 5-CH, 6-CH and PhCH2O). Crude alcohol 3b (7.06 g) was dissolved in anhydrous CH2Cl2 (330 mL) and the solution cooled to 0 °C. Triphenylphosphine (17.28 g, 0.066 mol) and carbon tetrabromide (21.85 g, 0.066 mol) were then added and the solution stirred at room temperature for 2 h. The reaction mixture was quenched with water and extracted with CH2Cl2. The organic phase was dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography on silica gel with hexanes/EtOAc (9/1) to yield 5.85 g (64percent) of bromide 3c. 1H NMR delta (CDCl3) 4.47 (s, 2H, PhCH2Br), 5.07 (s, 2H, PhCH2O), 6.90-7.46 (m, 9H, 2-CH, 4-CH, 5-CH, 6-CH and PhCH2O); 13C NMR (75 MHz) delta (acetone-d6) 32.5, 69.4, 114.3, 114.8, 121.0, 127.0 (2.x.), 127.2, 127.8, 128.0, 129.3, 136.1, 138.6, 158.3. |
| 56% |
With phosphorus tribromide; In diethyl ether; at 20℃; for 3h;Cooling with ice; |
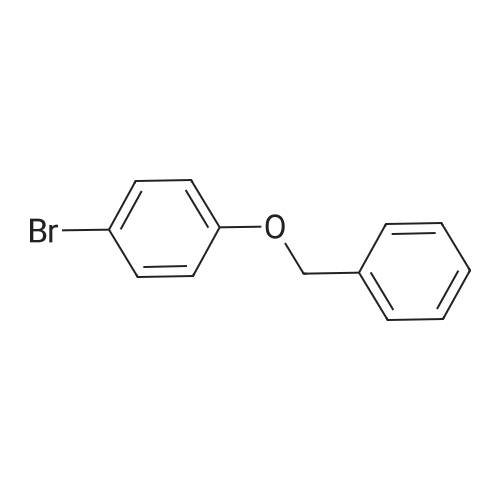
In an ice cooled solution of 3-benzyloxy-phenylmethanol (2.14 g, 10 mmol) in Et2O (25 mL), PBr333 (1.41 mL, 15 mmol) was added dropwise. The mixture was stirred at room temperature for 3 h and the reaction mixture was quenched by the addition of H2O (15 mL) in small portions at 0 °C. The aqueous phase was removed and the organic layer was washed with H2O, dried over Na2SO4 and evaporated in vacuo. The product was purified by column chromatography (silica gel) using petroleum ether (bp 40-60 °C)-EtOAc, 7:3 as eluent. Yield 1.5 g (56percent); yellow oil.1H NMR (CDCl3) delta: 7.42-6.82(m, 9H, Ph), 5.06(s, 2H, OCH2Ph), 4.46(s, 2H, BrCH2Ph).13C NMR delta: 159.0, 139.2, 136.7, 129.8, 128.6, 128.0, 127.5, 121.5, 115.4, 114.9 70.0(OCH2Ph), 33.4(BrCH2Ph).Anal. Calcd for C14H13BrO: C, 60.67; H, 4.73. Found: C, 60.46; H, 4.69. |
|
In hexane; dichloromethane; |
Example 57 3-benzyloxybenzylbromide A solution of 3-benzyloxybenzyl alcohol (9.54 g) in methylene chloride (40 ml) with TMSBr (2.5 eq, 13 mL) was heated at reflux for 1 hr. After cooling, the reaction was concentrated and then reconcentrated 2 X with methylene chloride (40 ml). The residue was chromatographed on silica gel eluding first with hexane followed by 10percent methylene chloride in hexane to afford 3-benzyloxybenzyl bromide which solidifies on cooling. 5.75 g m.p. 38°-40°. 1H NMR 500 MHz (CDCl3) d 7.43-7.3 (m, 5H), 7.22 (t, J=8.1 Hz, 1H), 7.0-6.9 (m, 2H), 6.88 (ddd, J=8.1, 2.4, 0.6 Hz), 5.021 (s, 2H), 4.417 (s, 2H) |
|
In hexane; dichloromethane; |
Example 57 3-benzyloxybenzylbromide A solution of 3-benzyloxybenzyl alcohol (2.04 g) in methylene chloride (20 mL) with TMS Bromide (2.5 eq. 3.14 mL) was heated at reflux for 6 hr. After cooling, the reaction was concentrated and then reconcentrated 2* from methylene chloride. The residue was chromatographed on silica gel with 5percent methylene chloride in hexane to afford 3-benzyloxybenzyl bromide (1.26 g) as an oil. 1 H NMR 500 MHz (CDCl3) d 7.43-7.3 (m, 5H), 7.22 (t, J=8.1 Hz, 1H), 7.0-6.9 (m, 2H), 6.88 (ddd, J=8.1, 2.4, 0.6 Hz, 1H), 5.021 (s, 2H), 4.417 (s, 2H) |
|
In hexane; dichloromethane; |
Example 57 3-benzyloxybenzylbromide A solution of 3-benzyloxybenzyl alcohol (9.54 g) in methylene chloride (40 ml) with TMSBr (2.5 eq, 13 mL) was heated at reflux for 1 hr. After cooling, the reaction was concentrated and then reconcentrated 2 * with methylene chloride (40 ml). The residue was chromatographed on silica gel eluding first with hexane followed by 10percent methylene chloride in hexane to afford 3-benzyloxybenzyl bromide which solidifies on cooling. 5.75 g m.p. 38°-40°. 1 H NMR 500 MHz (CDCl3) d 7.43-7.3 (m, 5H), 7.22 (t, J=8.1Hz, 1H), 7.0-6.9 (m, 2H), 6.88 (ddd, J=8.1, 2.4, 0.6 Hz), 5.021 (s, 2H), 4.417 (s, 2H) |
|
With carbon tetrabromide; triphenylphosphine; In dichloromethane; at 0 - 20℃; for 3h; |
Intermediate Ll. b.2 (Scheme 1.1) Step A: l-((3-(Bromomethyl)phenoxy)methyl)benzeneTo a solution of 3-benzyloxybenzyl alcohol (2 g, 9.3 mmol) and carbon tetrabromide (4 g, 12.1 mmol) in CH2Cl2 (70 mL), cooled to O°C, was added a solution of triphenylphosphine (2.9 g, 11.2 mmol) in CH2Cl2 (20 mL). The reaction was stirred at rt for 3 h and concentrated. Purification by flash chromatography (silica gel, 0-8percent EtOAc/hexanes) gave l-((3-(bromomethyl)phenoxy)methyl)benzene. 1H NMR (400 MHz, CDCl3) delta 7.39 (m, 5H), 7.25 (m, 1H), 7.00 (m, 2H), 6.92 (m, 1H), 5.06 (s, 2H), 4.46 (s, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping