| 186.1 mg |
|
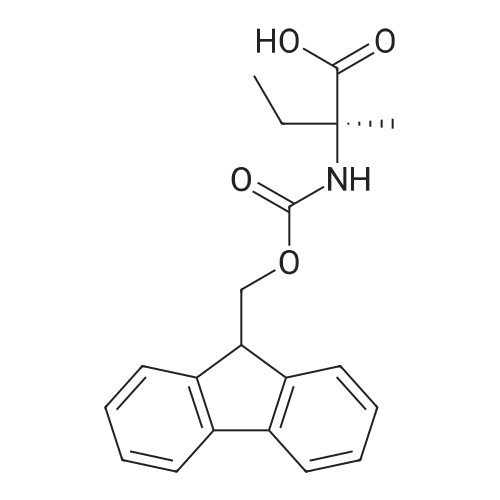
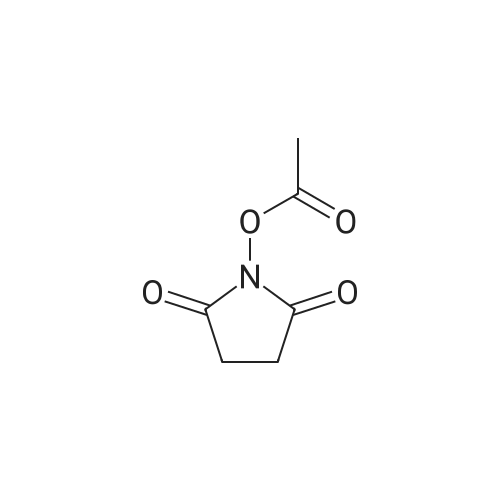
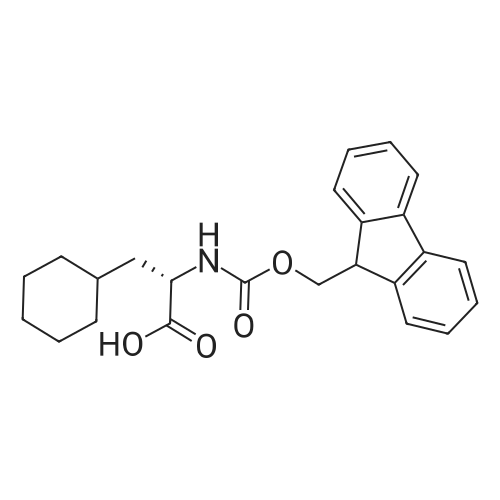
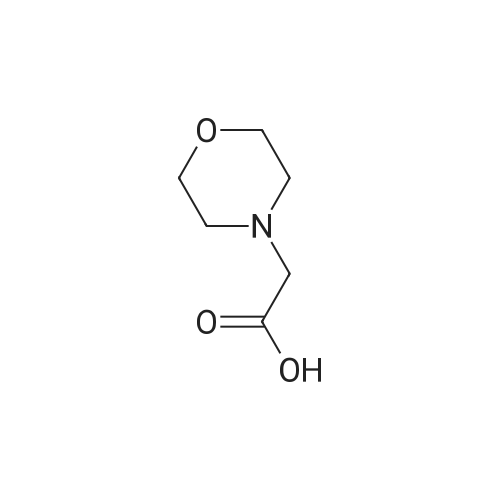
Example 29] (0337) (Synthesis method AC): Production of Ac-[D-Hyp24,Iva25,28,Pya(4)26,Cha27,36,Lys30,Aib31]-PYY(23-36) (compound No. 298) Compound No. 298: (0338) Synthesis of Ac-[D-Hyp24,Iva25,28,Pya(4)26,Cha27,36,Lys30,Aib31]-PYY (23-36) (0339) H-Asn(Trt)-Lys(Boc)-Aib-Thr(But)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Cha-Sieber Amide resin (SEQ ID NO:177) (952.8 mg, 0.25 mmol) obtained in Example 20 was weighed and placed in a reaction vessel, washed with DMF, and stirred in DMF for 20 min to swell the resin. Then, the resin was treated with Fmoc-Iva-OH (339.4 mg, 1 mmol), 0.5 M HOAt/DMF solution (2 mL, 1 mmol), DIPCDI (159 muL, mmol) for 120 min. The N-terminal Fmoc group was removed by 20percent piperidine/DMF treatment. By a similar procedure, Cha was introduced. In the same manner, removal of Fmoc group and condensation were repeated to introduce Pya(4), Iva, D-Hyp, Ser(But). After removal of Fmoc, the obtained resin was treated with AcOSu (157.1 mg, 1 mmol), DIEA (174.2 muL, 1 mmol) in DMF for 60 min, and washed with MeOH and dried to give Ac-Ser(But)-D-Hyp-Iva-Pya(4)-Cha-Iva-Asn(Trt)-Lys(Boc)-Aib-Thr(But)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Cha-Sieber Amide resin (1.1162 g). The obtained resin (1.1162 g) was treated with TFA: thioanisole: m-cresol: H2O: EDT: TIS (80:5:5:5:2.5:2.5) (6 mL) for 120 min, an operation to add diethyl ether to the reaction solution, precipitate a white powder by centrifugation, and remove diethyl ether by decantation was repeated twice. The residue was dissolved in aqueous acetic acid solution, passed through a disc filter with a pore diameter 0.45 mum to remove fine granules, and concentrated in an evaporator. After confirmation of the purity of the obtained crude peptide solution by HPLC, the peptide was purified by preparative HPLC in 6 portions using Daisopak-SP100-5-ODS-P 2×25 cm, and Solution A: 0.1percent TFA-water, Solution B: 0.1percent TFA-containing acetonitrile, flow rate 8 mL/min, A/B: 75/25-65/35 linear concentration gradient elution (60 min) was performed. The eluted object product was fractionated in test tubes, and each fraction was analyzed by HPLC to specify fractions containing only the object product. They were combined and freeze-dried to give 250.2 mg of a white powder. (0340) The obtained purified sample (250.2 mg, 140.47 mumol) was dissolved in water (20 mL), and AG 1x8 AcO resin (2.34 mL, 2.81 mmol equivalents) was added. The solution was stood for 1 hr while occasionally stirring with hand, passed through a disc filter with a pore diameter 0.45 mum to remove fine granules, concentrated in an evaporator to reduce the liquid amount to about 5 mL, and the solution was freeze-dried by cooling in a dry ice bath to give 186.1 mg of a white powder. MALDI-TOF-MS analysis, (M+H)1780.6 (Calculated 1781.1) HPLC elution time: 9.2 min elution condition (HPLC mode d): column: Merck Chromolith Performance RP-18e(4.6×100 mm I.D.) eluent: using Solution A: 0.1percent TFA-water, Solution B: 0.1percent TFA-containing acetonitrile, A/B: 80/20 - 30/70 linear concentration gradient elution (25 min) flow rate: 1.0 mL/min |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping