ATRP with ppb Concentrations of Photocatalysts
Coskun, Halil Ibrahim
;
De Luca Bossa, Ferdinando
;
Hu, Xiaolei
, et al.
JACS,2024,146(42):28994-29005.
DOI:
10.1021/jacs.4c09927
PubMed ID:
39388608
More
Abstract: In atom transfer radical polymerization (ATRP), dormant alkyl halides are intermittently activated to form growing radicals in the presence of a CuI/L/X-CuII/L (activator/ deactivator) catalytic system. Recently developed very active copper complexes could decrease the catalyst concentration to ppm level. However, unavoidable radical termination results in irreversible oxidation of the activator to the deactivator species, leading to limited monomer conversions. Therefore, successful ATRP at a low catalyst loading requires continuous regeneration of the activators. Such a regenerative ATRP can be performed with various reducing agents under milder reaction conditions and with catalyst concentrations diminished in comparison to conventional ATRP. Photoinduced ATRP (PhotoATRP) is one of the most efficient methods of activator regeneration. It initially employed UV irradiation to reduce the air-stable excited X-CuII/L deactivators to the activators in the presence of sacrificial electron donors. Photocatalysts (PCs) can be excited after absorbing light at longer wavelengths and, due to their favorable redox potentials, can reduce X-CuII/L to CuI/L. Herein, we present the application of three commercially available xanthene dyes as ATRP PCs: rose bengal (RB), rhodamine B (RD), and rhodamine 6G (RD-6G). Even at very low Cu catalyst concentrations (50 ppm), they successfully controlled PhotoATRP. Well-defined polymers with preserved livingness were prepared under green LED irradiation, with subppm concentrations ([PC] ≥ 10 ppb) of RB and RD-6G or 5 ppm of RD. Interestingly, these PCs efficiently controlled ATRP at wavelengths longer than their absorption maxima but required higher loadings. Polymerizations proceeded with high initiation efficiencies, yielding polymers with narrow molecular weight distributions and high chain-end fidelity. UV?vis, fluorescence, and laser flash photolysis studies helped to elucidate the mechanism of the processes involved in the dual-catalytic systems, comprising parts per million of Cu complexes and parts per billion of PCs.
Purchased from AmBeed:
16858-01-8 ;
33527-91-2

Grafting well-defined polymers onto unsaturated PVDF using thiol-ene reactions
Ting-Chih Lin
;
Piotr Mocny
;
Martin Cvek
, et al.
Polymer,2024,126848.
DOI:
10.1016/j.polymer.2024.126848
More
Abstract: Poly(vinylidene fluoride) (PVDF) is commonly used in membranes, lithium-ion battery binders, and coatings due to its thermal and chemical robustness. Nevertheless, PVDF-based copolymers can broaden the application scope and performance capabilities of pristine PVDF. PVDF has been modified via grafting-from reactions. However, grafting density and graft length, two important properties of graft copolymers, cannot be accurately determined. Herein, we used grafting-onto thiol-ene reactions as a method to modify PVDF. The molar mass of pre-synthesized, thiol-terminated polymers were accurately determined, and grafting densities were calculated. Unsaturated sites were generated through dehydrofluorination and dehydrochlorination in PVDF and P(VDF-co-chlorotrifluoroethylene) (PVDF-CTFE). Various conditions were studied, including the molar mass and chemical structure of grafts, the degree of thiol substitution, and thiol-ene reaction mechanisms. Base-catalyzed Michael addition with secondary thiols performed best, with the highest grafting density calculated to be about 4 chains per PVDF chain. Despite the low grafting density, changes in material properties between the product and starting materials were observed, validating this controlled method for PVDF modification.
Keywords:
poly(vinylidene fluoride) ;
Controlled radical polymerization ;
Thiol-eneGrafting-onto
Purchased from AmBeed:
16858-01-8 ;
7789-45-9

Tuning Polymer Composition Leads to Activity-Stability Tradeoff in Enzyme-Polymer Conjugates
Bisirri, Evan A.
;
Wright, Thaiesha A.
;
Schwartz, Daniel K.
, et al.
Biomacromolecules,2023,24(9):4033-4041.
DOI:
10.1021/acs.biomac.3c00396
PubMed ID:
37610792
More
Abstract: Protein-polymer conjugation provides an opportune means to adjust the local environment of proteins and enhance protein stability, performance, and solubility Although much attention has been focused on tuning protein-polymer interactions, the properties of polymer-modified proteins may also be altered by polymer-polymer interactions. Herein, we sought to better understand the influence of polymer-polymer interactions on Candida rugosa lipase, which was modified with random co-polymers composed of sulfobetaine methacrylate (SBMA) and poly(ethylene glycol) methacrylate (PEGMA). Our findings suggest that there is an apparent activity-stability tradeoff as a function of polymer composition Specifically, as the ratio of SBMA to PEGMA increased, lipase stability was enhanced, whereas activity decreased. By tuning the monomer ratio, we showed that lipase productivity could be optimized. These findings are discussed in the context of complex enzyme-polymer and polymer-polymer interactions and ultimately may enable more informed conjugate designs and improved enzyme productivity in industrial biotransformations under harsh or extreme conditions.
Purchased from AmBeed:
16858-01-8

Highly Sensitive Detection of Bacteria by Binder-Coupled Multifunctional Polymeric Dyes
Kapil, Kriti
;
Xu, Shirley
;
Lee, Inseon
, et al.
Polymers,2023,15(12):2723.
DOI:
10.3390/polym15122723
PubMed ID:
37376368
More
Abstract: Infectious diseases caused by pathogens are a health burden, but traditional pathogen identification methods are complex and time-consuming. In this work, we have developed well-defined, multifunctional copolymers with rhodamine B dye synthesized by atom transfer radical polymerization (ATRP) using fully oxygen-tolerant photoredox/copper dual catalysis. ATRP enabled the efficient synthesis of copolymers with multiple fluorescent dyes from a biotin-functionalized initiator. Biotinylated dye copolymers were conjugated to antibody (Ab) or cell-wall binding domain (CBD), resulting in a highly fluorescent polymeric dye-binder complex. We showed that the unique combination of multifunctional polymeric dyes and strain-specific Ab or CBD exhibited both enhanced fluorescence and target selectivity for bioimaging of Staphylococcus?aureus by flow cytometry and confocal microscopy. The ATRP-derived polymeric dyes have the potential as biosensors for the detection of target DNA, protein, or bacteria, as well as bioimaging.
Keywords:
pathogen identification ;
bioimaging ;
fluorescence ;
copolymer ;
ATRP ;
flow cytometry ;
confocal imaging
Purchased from AmBeed:
16858-01-8

Visible Light-ATRP Driven by Tris(2-Pyridylmethyl)Amine (TPMA) Impurities in the Open Air
Jazani, Arman Moini
;
Schild, Dirk J.
;
Sobieski, Julian
, et al.
Macromol. Rapid Comm.,2023,44(16):2200855.
DOI:
10.1002/marc.202200855
PubMed ID:
36471106
More
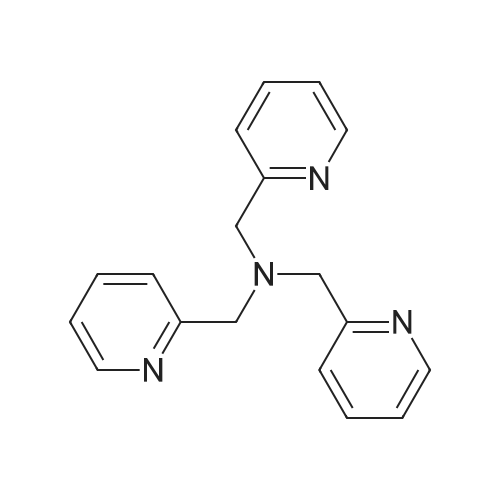
Abstract: Atom transfer radical polymerization (ATRP) of oligo(ethylene oxide) monomethyl ether methacrylate (OEOMA500) in H2O is enabled using CuBr2 with tris(2-pyridylmethyl)amine (TPMA) as a ligand under blue or green-light irradiation without requiring any addnl. reagent, such as a photo-reductant, or the need for prior deoxygenation. Polymers with low dispersity (D = 1.18-1.25) are synthesized at high conversion (>95%) using TPMA from 3 different suppliers, while no polymerization occurred with TPMA synthesized and purified in the laboratory Based on spectroscopic studies, probably TPMA impurities (i.e., imine and nitrone dipyridine), which absorb blue and green light, can act as photosensitive co-catalyst(s) in a light region where neither pure TPMA nor [(TPMA)CuBr]+ absorbs light.
Keywords:
ATRP ;
green light ;
oxygen tolerance ;
water
Purchased from AmBeed:
16858-01-8
Amine (TPMA) Impurities in the Open Air.png)
Reductive Termination of Cyanoisopropyl Radicals by Copper(I) Complexes and Proton Donors: Organometallic Intermediates or Coupled Proton-Electron Transfer?
Thevenin, Lucas
;
Fliedel, Christophe
;
Fantin, Marco
, et al.
Inorg. Chem.,2019,58(9):6445-6457.
DOI:
10.1021/acs.inorgchem.9b00660
PubMed ID:
30990024
More
Abstract: Cyanoisopropyl radicals, generated thermally by the decomposition of azobis(isobutyronitrile) (AIBN), participate in reductive radical termination (RRT) under the combined effect of copper(I) complexes and proton donors (water, methanol, triethylammonium salts) in acetonitrile or benzene. The investigated copper complexes were formed in situ from [CuI(MeCN)4]+BF4- in CD3CN or CuIBr in C6D6 using tris[2-(dimethylamino)ethyl]amine (Me6TREN), tris(2-pyridylmethyl)amine (TPMA), and 2,2'-bipyridine (BIPY) ligands. Upon keeping all other conditions constants, the impact of RRT is much greater for the Me6TREN and TPMA systems than for the BIPY system. RRT scales with the proton donor acidity (Et3NH+ ? H2O > CH3OH), it is reduced by deuteration (H2O > D2O and CH3OH > CD3OD), and it is more efficient in C6D6 than in CD3CN. The collective evidence gathered in this study excludes the intervention of an outer-sphere proton-coupled electron transfer (OS-PCET), while an inner-sphere PCET (IS-PCET) cannot be excluded for coordinating proton donors (water and methanol). On the other hand, the strong impact of RRT for the noncoordinating Et3NH+ in CD3CN results from the formation of an intermediate CuI-radical adduct, suggested by DFT calculations to involve binding via the N atom to yield keteniminato [L/Cu-N:C:CMe2]+ derivatives with only partial spin delocalization onto the Cu atom.
Keywords:
Copper ;
Coupled Proton-Electron Transfer ;
Protonolysis ;
Organocopper(II) complexes ;
Radical
Purchased from AmBeed:
16858-01-8
 Complexes and Proton Donors Organometallic Intermediates or Coupled Proton-Electron Transfer.png)

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







Amine (TPMA) Impurities in the Open Air.png)
 Complexes and Proton Donors Organometallic Intermediates or Coupled Proton-Electron Transfer.png)