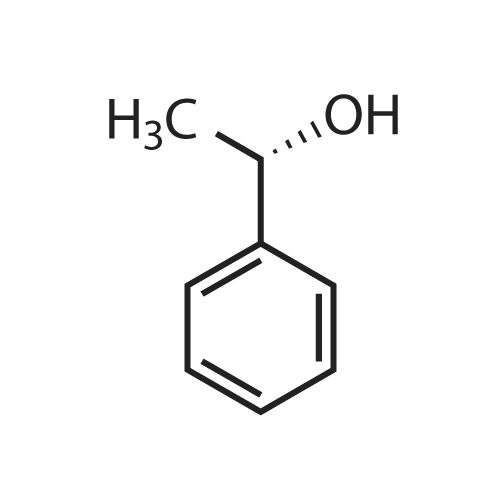
| 92% |
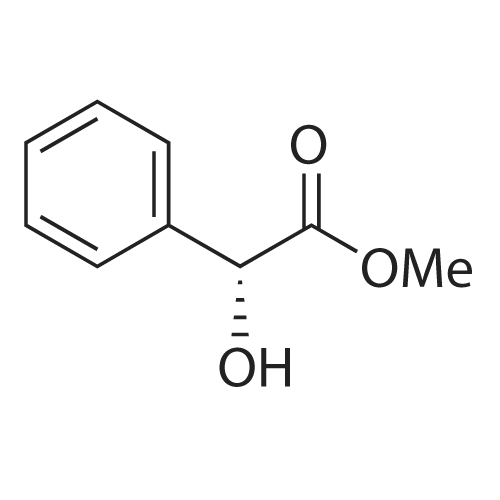
With potassium osmate dihydrate; potassium carbonate; (9S,9"S)-9,9"-[phthalazine-1,4-diylbis-(oxy)]bis[10,11-dihydro-6'-methoxycinchonane]; potassium hexacyanoferrate(III); In water; tert-butyl alcohol;Electrochemical reaction; Irradiation; Inert atmosphere; |
General procedure: A flame-dried three-necked round-bottomed flask was chargedunder argon with 1 equiv of o-phenylenediamine (0.31 mmol,33.8 mg), 1.1 equiv of 3-nitrobenzaldehyde (0.34 mmol,52 mg), 20 mol % CAN (31 mg), and LiClO4 (0.1 M, 127 mg)in 12 mL of THF/MeOH 5:1. A RVC anode and a carbon rodcathode were inserted into the flask. A constant current of 8 mAwas supplied (either via a potentiostat or a photovoltaic cell)until 2.3 F/mol of charge had passed. After the electrolysis, thecontents of the flask were extracted with EtOAc, washed withbrine, and dried with MgSO4. The product was purified bycolumn chromatography (EtOAc/hexanes 1:1) to give the benzimidazoleproduct |
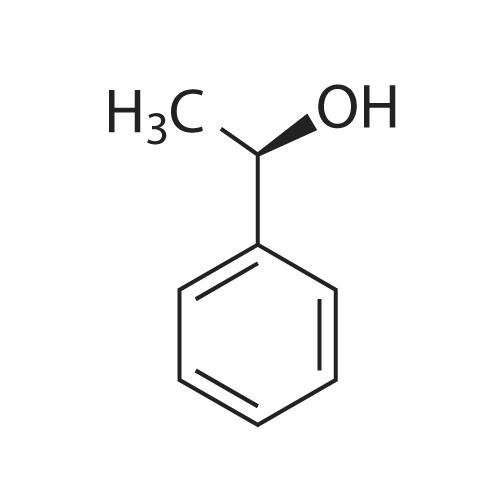
| 82% |
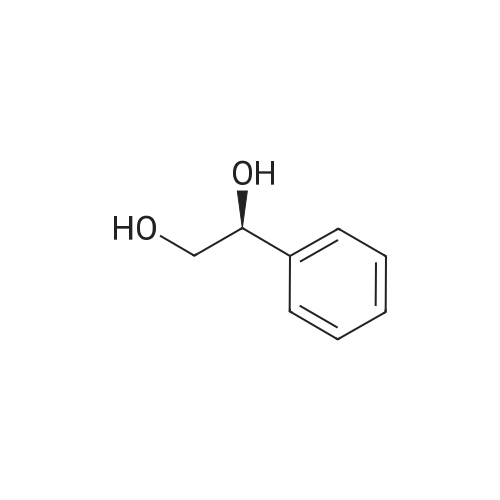
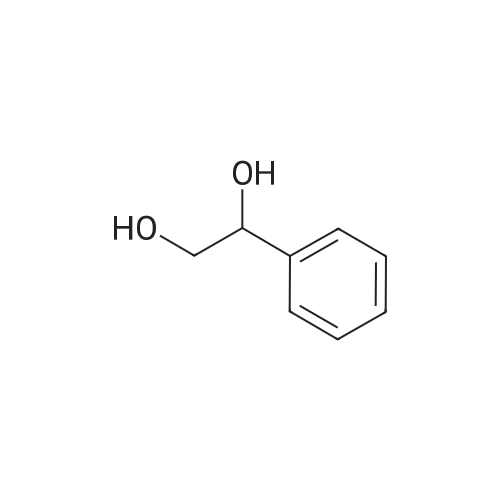
With dihydrogen peroxide; In toluene; at 55℃; for 1.8h;Green chemistry; |
Take a certain amount of styrene, followed by adding its mass is 0.3 times and 1.2 times the catalyst 40% hydrogen peroxide, reaction at 55 1.8h, to obtain a reaction mixture;The method of preparation of the catalyst was: S- take a certain amount of nicotine, which was added 2.25 times mass of bromooctadecane, 115 refluxed in toluene for 20 hours, the solvent evaporated under reduced pressure to give a yellow viscous chiral ionic liquids , was added dropwise with stirring a solution of phosphorous acid to a concentration of 6% by mass percentage of, 400r / min, the quality of phosphate acid solution stirring rate was 20 times the mass of S- nicotine, generating a particle diameter of 0.4mm shaped yellow precipitation, filtration and drying to obtain a catalyst;The toluene was used in an amount 12 times the mass of S- nicotine;2) a) step the reaction mixture was added to 18 times its mass of water at room temperature, filtered, and recovered the precipitate, the filtrate was added 8 times the mass of ethyl acetate, the extracts were collected, the solvent was evaporated under reduced pressure to give (R) - benzene white glycol base crude;3) Step 2) The crude product was dissolved in 0.2 times its mass, petroleum ether 75 , the cooling was recrystallized, filtered and dried to obtain (R) - phenyl glycol pure.Obtained by the method described above (R) - phenyl ethylene glycol in a yield of 82%, compared with the standard of enantiomeric excess (ee) of 100%. |
| 80% |
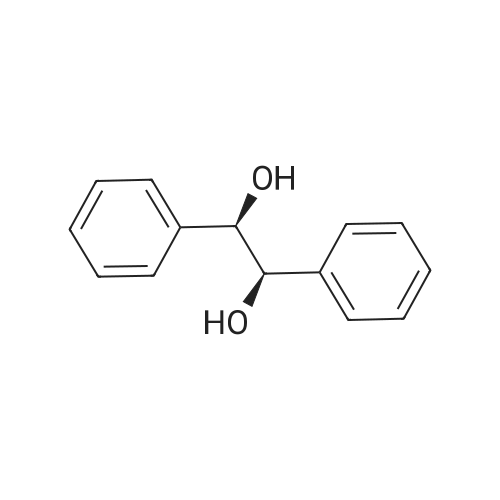
With potassium osmate(VI); disodium hydrogenphosphate; dipotassium peroxodisulfate; methanesulfonamide; (9S,9"S)-9,9"-[phthalazine-1,4-diylbis-(oxy)]bis[10,11-dihydro-6'-methoxycinchonane]; potassium hexacyanoferrate(III); In water; tert-butyl alcohol; at 20℃; |
General procedure: Water (5 mL/mmol substrate) was added to a solid mixture of K2S2O8 (1.5 equiv), Na2HPO4 (3 or 4 equiv), NaIO4 (0.2 equiv) or K3Fe(CN)6 (0.2 equiv), MeSO2NH2 (1 equiv) and K2OsO2(OH)4 (0.05 equiv) at room temperature, and the mixture was stirred for 5 min. (DHQD)2Phal (0.075 equiv), tert-BuOH (5 mL/mmol substrate), and the olefin (1 equiv) were then added sequentially, and the reaction was stirred at room temperature until olefin was consumed as judged by TLC. A solution of saturated aqueous Na2S2O3 was added, and the mixture was extracted with CH2Cl2 (3 x 5 mL/mmol substrate).The combined organic extracts were dried (Na2SO4), filtered, and concentrated under reduced pressure, and the crude product was purified by flash chromatography to provide the pure diol. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping