| 100% |
|
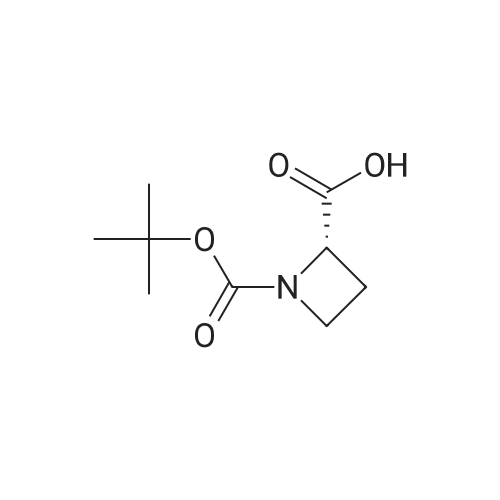
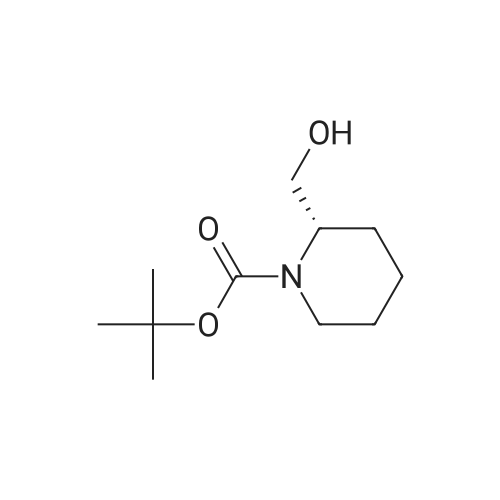
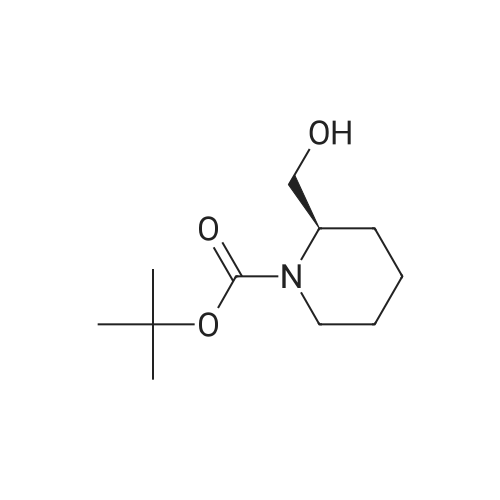
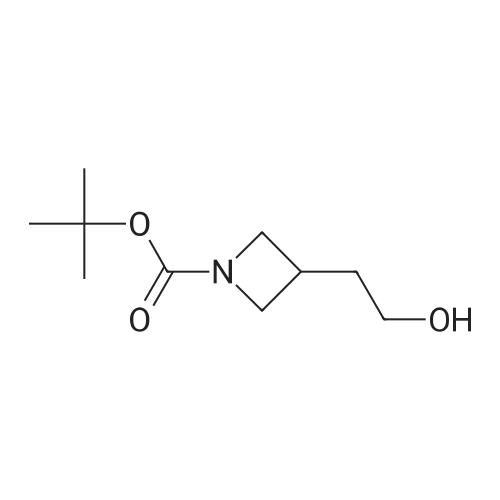
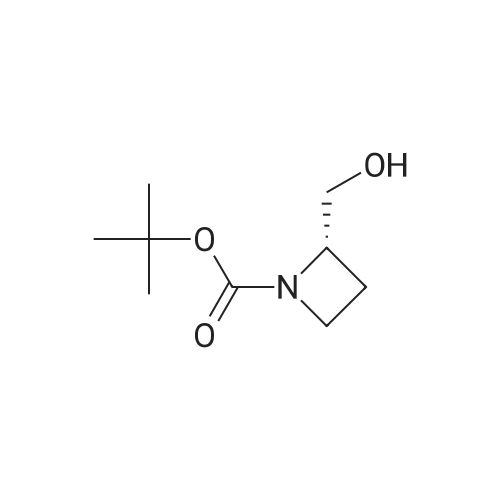
Example 16 (S)-tert-Butyl 2-(Hydroxymethyl)azetidine-1-carboxylate (14).17 To a solution of 13 (0.94 g, 4.7 mmol) in THF (10 mL) was added slowly a 1 M BH3 in THF (21.0 mL) at 0 C. The mixture was stirred 2 days at ambient temperature, then cold water (20 mL) was added at 0 C. After evaporation of the THF in vacuo, an 10% aqueous solution of citric acid (15 mL) was added and extracted with ethyl acetate (50 mL*2). The combined ethyl acetate was washed with saturated NaHCO3 (30 mL) and NaCl (30 mL), and dried over Na2SO4. Evaporation of the ethyl acetate in vacuo afforded 0.86 g (100%) of 14 as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 4.40 (m, 1), 3.85-3.70 (m, 3H), 2.13 (m, 1H), 1.90 (m, 1H), 1.42 (s, 9H). |
| 87% |
With boron dimethyl-trifluoro sulphide; In tetrahydrofuran; at 0℃; for 2h;Heating / reflux; |
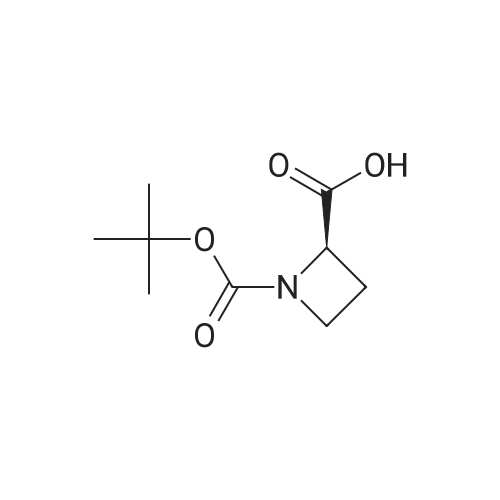
(S)-1-(tert-butoxycarbonyl)-2-azetidinecarboxylic acid (10.42 g, 49.4mmol) was introduced into a 500-mLpear-shaped flask, and tetrahydrofuran (200 mL) was added to dissolve the compound. Then, a tetrahydrofuran solution of 10 M borane-dimethyl sulfide complex salt (9.87 mL, 98.7 mmol) was slowly added at 0C, and the mixture was heated to reflux for 2 hours while stirring. The reaction solution was left to cool, and then was concentrated under reduced pressure. Ice water (100 mL) was poured thereto, and the mixture was extracted with ethyl acetate (200 mLx2). The extract was washed with saturated brine (200 mL), and then dried over anhydrous sodium sulfate. After filtering the extract, the filtrate was concentrated under reduced pressure to obtain the title compound (8.03 g, 42.9mmol, 87%) as an oily matter. NMR(CDCl3)δ:1.45(9H,s),1.93(1H,brs),2.13-2.22(1H,m),3.67-3.81(3H,m),3.87(1H,q,J=8.8Hz),4.21(1H,br s),4.44(1H,br s). MS(ESI)m/z:188(M++1). |
|
With sodium tetrahydroborate; iodine; In tetrahydrofuran; at 0℃; for 19.5h;Reflux; Inert atmosphere; |
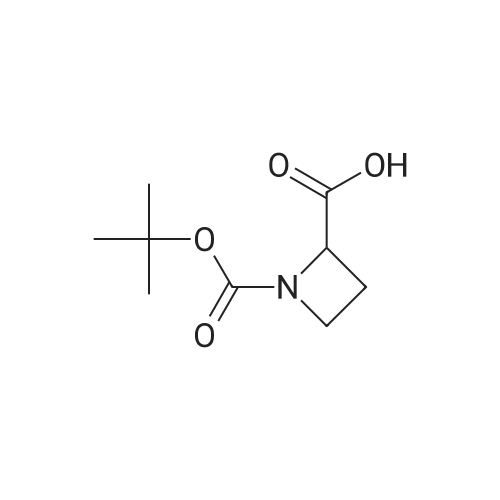
To 699 mg (18.48 mmol) sodium borohydride in 20 mL dry THF under nitrogen, was added 1.15 g (5.74 mmol) (S)-azetidine-1 ,2-dicarboxylic acid 1 -te/f-butyl ester and the reaction was cooled to 0 C, 1.72 g (6.78 mmol) iodine in 10 mL dry THF was added dropwise over 30 minutes, the reaction mixture was allowed to warm to room temperature, and was heated under reflux for 19 hours. The mixture was cooled to room temperature and 25 mL MeOH was added dropwise over 15 minutes. After evaporation of the solvent, 30 mL KOH (1 M) was added and the mixture was stirred at room temperature for 2 hours. The aqueous phase was extracted with DCM (2 x 75 mL) and the combined organic layers were washed with saturated brine (3 x 50 mL), then dried over MgSO4. After filtration, the solvent was evaporated to give the desired product, which was used for the next step without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping