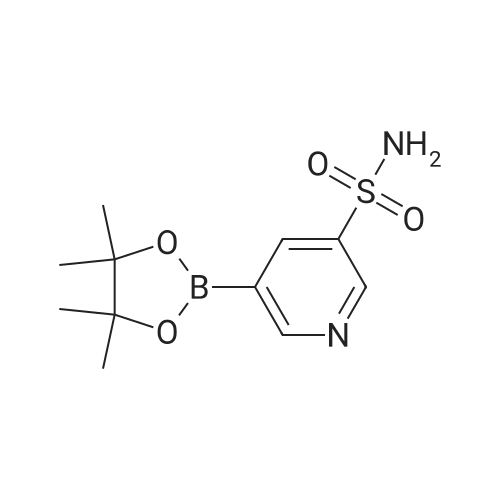
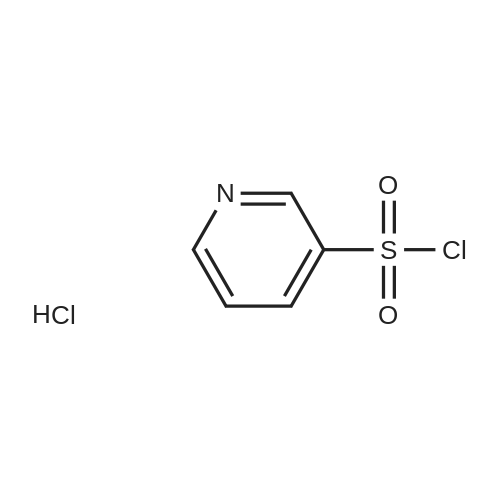
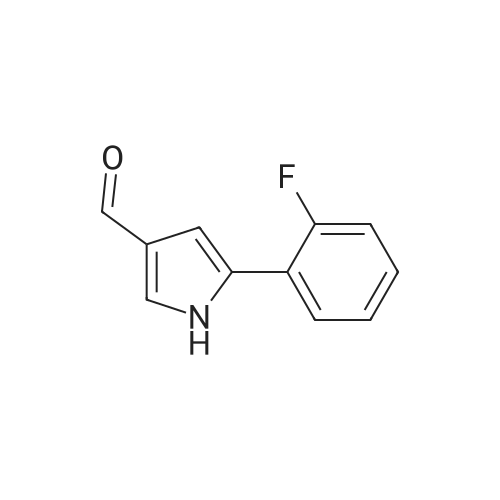
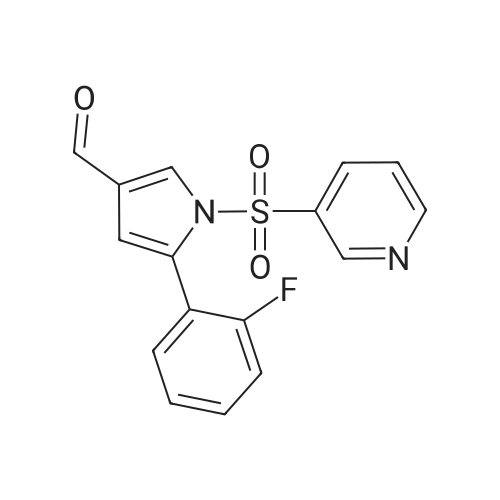
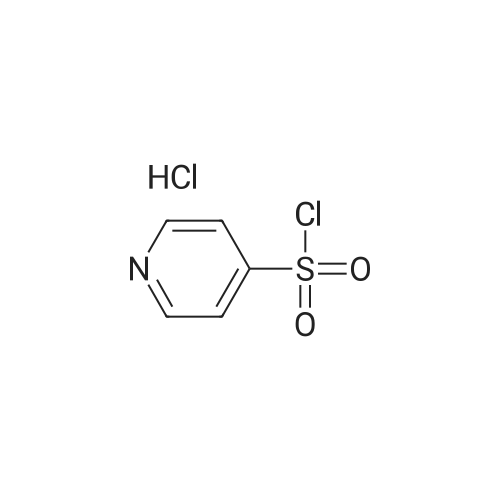
| 87% |
With dmap; N-ethyl-N,N-diisopropylamine; In acetonitrile; at 40 - 50℃; for 1h; |
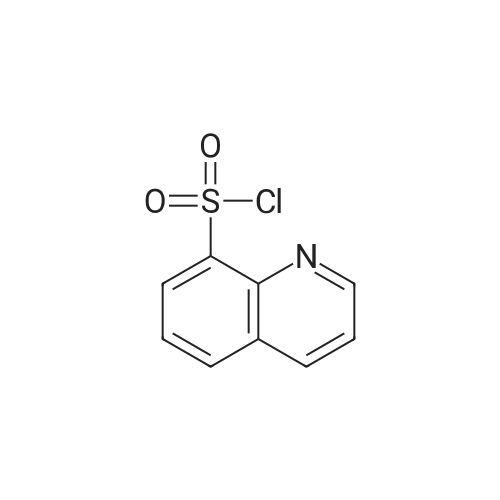
5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde (7.0 g, 37.00 mmol), N,N-dimethylpyridine-4-amine (0.902 g, 7.38 mmol), diisopropylethylamine (6.69 g, 51.80 mmol) and acetonitrile (28 mL) were added to a four-necked flask, then pyridine-3-sulfonyl chloride (7.89 g, 44.42 mmol) was added dropwise, and the mixture was washed well with acetonitrile (3.5 mL). The mixture was stirred at an inside temperature of 40-50 C. for about 1 hr, and cooled to an inside temperature of 25-35 C., and water (21 mL) was added dropwise at the same temperature. Then the mixture was adjusted to pH 4-5 at room temperature with 0.5N hydrochloric acid (8 mL), and water (41 mL) was added dropwise at room temperature. After stirring at room temperature for 30 min, the inside temperature was cooled to 0-10 C., and the mixture was stirred for 1 hr. The precipitated crystals were collected by filtration, washed with acetonitrile (14 mL)/water (28 mL) cooled to 5 C., and dried under reduced pressure at 50 C. until a constant weight was reached to give the title compound (10.6 g, yield 87.0%). 1H-NMR (CDCl3, TMS, 500 MHz) delta (ppm): 6.68 (d, J=1.6 Hz, 1H), 7.02 (dd, J=8.2, 8.2 Hz, 1H), 7.14-7.19 (m, 2H), 7.38 (dd, J=8.2, 4.9 Hz, 1H), 7.44-7.48 (m, 1H), 7.72 (ddd, J=8.2, 2.5, 1.6 Hz, 1H), 8.17 (d, J=1.9 Hz, 1H), 8.59 (d, J=1.9 Hz, 1H), 8.82 (dd, J=4.7, 1.6 Hz, 1H), 9.90 (s, 1H). |
| 86.7% |
With N-ethyl-N,N-diisopropylamine;dmap; In acetonitrile; at 40 - 50℃; for 1.5h; |
Example 4 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde (5.00 g, 26.43 mmol), N,N-dimethylpyridin-4-amine (0.65 g, 5.29 mmol), diisopropylethylamine (4.78 g, 37.00 mmol) and acetonitrile (18.5 ml) were added in a four neck flask, and a solution of pyridine-3-sulfonyl chloride (5.63 g, 31.71 mmol) in acetonitrile (5 ml) was added. Acetonitrile (1.5 ml) was further added, and the mixture was stirred at the internal temperature of 40-50 C. for 1.5 hr. The internal temperature was cooled to 30 C., and water (15 ml) was added dropwise. The mixture was adjusted to pH 4-5 with 0.5 N hydrochloric acid. Seed crystals (2.5 mg) of the title compound were added, and then water (about 30 ml) was added dropwise. After stirring at the internal temperature of 20-30 C. for 0.5 hr, the internal temperature was cooled to 0-10 C., and the mixture was stirred for 1 hr. The precipitated crystals were collected by filtration, washed with a cold mixed solution of acetonitrile and water (1:2, 7.5 ml), and water (7.5 ml*2), and dried under reduced pressure at 50 C. until a constant weight was reached to give the title compound (7.57 g, yield 86.7%). 1H-NMR (300 MHz, CDCl3) delta (ppm): 6.68 (d, J=1.7 Hz, 1H), 7.01-7.05 (m, 1H), 7.16-7.18 (m, 2H), 7.37-7.40 (m, 1H), 7.45-7.51 (m, 1H), 7.69-7.72 (m, 1H), 8.15 (d, J=1.8 Hz, 1H), 8.58 (d, J=1.7 Hz, 1H), 8.82 (dd, J=4.8, 1.5 Hz, 1H), 9.90 (s, 1H). elemental analysis (C16H11N2O3SF). Calculated: C, 58.17; H, 3.36; N, 8.48; O, 14.53; S, 9.71; F, 5.75.Found: C, 58.32; H, 3.46; N, 8.54; S, 9.76; F, 5.62.melting point 106-108 C. |
| 86.6% |
With dmap; triethylamine; In dichloromethane; for 2h; |
10.00 g of <strong>[881674-56-2]5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde</strong>, 1.29 g of 4-dimethylaminopyridine (DMAP), 7.49 g of triethylamine, and 1 L of dichloromethane were put into the reactor, and the temperature was reduced by stirring. 11.26 g of pyridine-3-sulfonyl chloride was added dropwise, and the reaction was stirred for 2 hours after the dropwise addition.Add water 20ml quench reaction, followed by 20ml of water, the mass percentage concentration of 20% sodium chloride solution 20ml washing, distillation, add 70ml 75% ethanol solution to heat the solution, cooling crystallization, filtration, washing, drying to obtain 1-(3- Pyridinesulfonyl)-<strong>[881674-56-2]5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde</strong> 15.12 g.Yield 86.60%. |
| 86.0% |
With dmap; triethylamine; In acetonitrile; at 50℃;Large scale; |
Add 2.5kg of 5- (2-fluorophenyl) -1H-pyrrole-3-formaldehyde to a clean 50L reaction kettle,4-dimethylaminopyridine 0.323kg, triethylamine 1.60kg and acetonitrile 7.31kg,A solution of pyridine-3-sulfonyl chloride in acetonitrile was added dropwise with stirring (2.82 kg of pyridine-3-sulfonyl chloride was added with 2.0 kg of acetonitrile). After the dropwise addition was completed, the temperature was raised to 50 C, and the reaction was kept warm.The reaction progress was monitored by TLC (developing agent: PE: EA = 2: 1). After the reaction was completed, the temperature was lowered to 25 C, and 7.5 kg of purified water was added dropwise.Add 0.5 mol / L hydrochloric acid dropwise to adjust the pH of the system to 4, and then dropwise add 15.0 kg of purified water, stir at 25 C for 0.5h, lower the temperature to 10 C, and stir for 1h.After filtration, the filter cake was rinsed with a mixed solution of 1.0 kg of acetonitrile and 2.5 kg of purified water and 7.5 kg of purified water.Drying gave 5- (2-fluorophenyl) -1- (pyridin-3-ylsulfonyl) -1H-pyrrole-3-carboxaldehyde 3.75 kg with a yield of 86.0%. |
| 78.39% |
With dmap; triethylamine; In dichloromethane; at 10 - 35℃; for 3h; |
Put 50.03 g of 5- (2-fluorophenyl) -1H-pyrrole-3-carboxaldehyde into the reaction tank, and add 6.47 g of 4-N, N-dimethylaminopyridine (DMAP), 37.78 g of triethylamine, and dichloro 250 ml of methane, stirred, 56.41 g of pyridine-3-sulfonyl chloride was added dropwise at an internal temperature of 10 to 35 C, and the internal temperature was maintained at 20 ± 5 C after the dropwise reaction. The TLC was monitored to 5- (2-fluorophenyl) -1H-pyrrole- 3- After the basic reaction of formaldehyde is complete (about 3h), add stirring to quench the reaction, separate the liquid, wash it once with water, evaporate to a distillate under reduced pressure, add 90% ethanol, heat, dissolve at reflux for 30min, cool down, and internal temperature 0 Stir and crystallize at -5 C for 2h, filter, wash with 50% ethanol solution, drain, dry at 60-80 C for 3h, weigh to obtain 5- (2-fluorophenyl) -1- (pyridin-3-ylsulfonyl) 68.48 g of -1H-pyrrole-3-carboxaldehyde, yield 78.39%. |
| 68% |
With lithium hexamethyldisilazane; In tetrahydrofuran; at 0 - 20℃; for 1.08h; |
200 mg of intermediate VI-1 was dissolved in 15 ml of anhydrous tetrahydrofuran, and the temperature was lowered to 0 C, then 1.3 ml of 1 M bistrimethylsilylamide lithium was added dropwise, and the reaction was continued at 0 C after the addition was completed. After a minute, 213 mg of pyridine-3-sulfonyl chloride was further added, followed by incubation at 0 C for 5 minutes, and then the reaction was continued to room temperature for 1 hour. After completion of the reaction, the reaction was quenched by the addition of sodium hydrogen carbonate solution, and the mixture was evaporated to dryness. column chromatography to give the compound of formula VII-1, 237 mg of a white solid, yield 68% |
| 15.5 g |
With dmap; N-ethyl-N,N-diisopropylamine; In acetonitrile; at 40 - 50℃; for 2h; |
Acetonitrile (50 ml) was added to the reaction flask at room temperature,5- (2-fluorophenyl) -1H-pyrrole-3-carbaldehyde (10 g)4-dimethylaminopyridine (1.3 g)And N, N-diisopropylethylamine (13 g),40 ~ 50 stirring reaction 2 hours after the thin layer chromatography to complete the reaction.Adding 1mol / L hydrochloric acid solution to the reaction system to adjust pH = 4 ~ 5,Add water (60ml) and stir.filter,dry,To give 5- (2-fluorophenyl) -1 - [(pyridin-3-yl) sulfonyl] -1H-pyrrole-3-carbaldehyde(15.5 g, yellow solid). |
| 15 g |
With triethylamine; In acetonitrile; at 45℃; for 1.5h; |
5- (2-fluorophenyl) pyrrole-3-carbaldehyde 10g, 4- dimethylaminopyridine 1.3g, triethylamine 7.5g and acetonitrile (40ml) added to the reaction flask, stirred at room temperature; pyridine-3-sulfonyl chloride 11.3g and acetonitrile (10ml), the reaction flask was added dropwise; the reaction was heated to 45 1.5 hours; cooled to 25 , was added water (30ml); the system with concentrated hydrochloric acid adjusted to ph 4-5, stirred for half an hour at 25 deg.] C; was cooled to 0-5 deg.] C stirred for 1 hour; the filter cake with acetonitrile: water (1: 2) 30ml rinsed again with water (20ml) was rinsed 2 times, 50 deg.] C and dried in vacuo to give 5- (2-fluorophenyl yl) -1- (pyridin-3-sulfonyl) pyrrole-3-carbaldehyde 15g; |
| 0.83 mol |
With dmap; sodium carbonate; In acetonitrile; at 60℃; for 5h; |
Weigh 1 mol of pyridine-3 sulfonyl chloride and 1 mol of <strong>[881674-56-2]5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde</strong> in an appropriate amount of acetonitrile solvent, and add 1 mol of an acid-binding agent, and the acid-binding agent is DMAP and The composition of sodium carbonate was uniformly stirred, heated to 60 C, and the temperature was raised to 1 C / min. The temperature was raised to 60 C and then incubated for 5 hours to carry out a nucleophilic substitution reaction. The reaction formula of the nucleophilic substitution reaction is as follows: As shown in the above reaction formula, the product of the nucleophilic substitution reaction is 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde, said 5-(2- Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde is still present in the acetonitrile solvent. Next, the mixture after the above nucleophilic substitution reaction was allowed to stand for 3 h, and gradually cooled to room temperature.The mixture will undergo crystallization during the cooling process to form a solid-liquid mixture, and then the filtration operation is performed.The solid obtained after filtration is the nucleophilic substitution reaction product 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde.It was dried, weighed, and converted to obtain 0.83 mol of 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +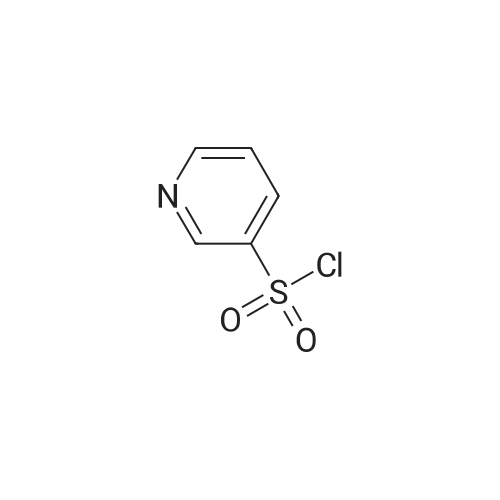

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping