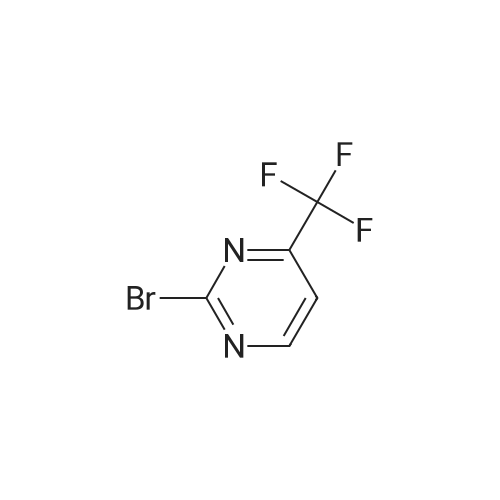
| 82% |
Stage #1: With N-Bromosuccinimide In chloroform for 20 h;
Stage #2: With sodium hydroxide In dichloromethane; chloroform; water |
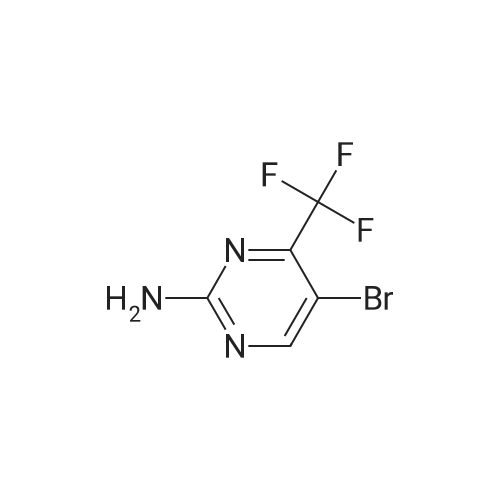
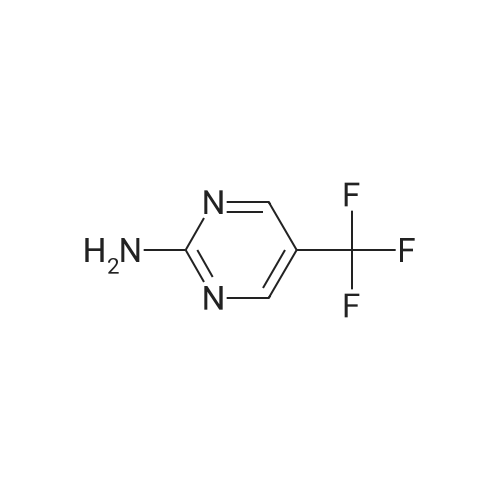
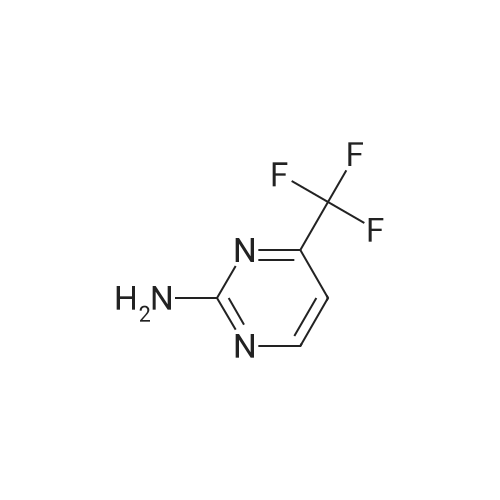
[0250] To a solution of 2-amino-4-trifluoromethylpyrimidine (8.0 g, 49.1 mmol) in chloroform (300 mL) was added N-bromosuccinimide (8.9 g, 50 mmol). The solution was stirred in the dark for 16 hours, at which time additional N-bromosuccinimide (4.0 g, 22.5 mmol) was added. After stirring for an additional 4 hours the solution was added to CH2CI2 (200 mL) and IN NaOH (200 mL). Upon mixing, the layers were separated and <n="97"/>the organic layer was washed with NaCl(sat.) (100 mL), dried over Na2SO4, filtered and concentrated, yielding 10.9 g (82percent) of 5-bromo-4-(trifluoromethyl)-2-pyrimidylamine. LCMS (m/z): 242/244 (MH+). 1H NMR (CDCl3): δ 8.52 (s, IH), 5.38 (bs, 2H). |
| 82% |
Stage #1: With N-Bromosuccinimide In chloroform for 20 h; Darkness
Stage #2: With sodium hydroxide In dichloromethane; chloroform |
[0092] Synthesis -bromo-4-(trifluoromethyl)pyrimidin-2-amine[0093] To a solution of 2-amino-4-trifluoromethylpyrimidine (8.0 g, 49.1 mmol) in chloroform (300 mL) was added N-bromosuccinimide (8.9 g, 50 mmol). The solution was stirred in the dark for 16 hours, at which time additional N-bromosuccinimide (4.0 g, 22.5 mmol) was added. After stirring for an additional 4 hours the solution was added to CH2C12 (200 mL) and IN NaOH (200 mL). Upon mixing, the layers were separated and the organic layer was washed with NaCl(sat.) (100 mL), dried over Na2S04, filtered and concentrated, yielding 10.9 g (82percent) of 5-bromo-4-(trifluoromethyl)-2-pyrimidylamine. LCMS im/z): 242/244 (MH ). 3/4 NMR (CDC13): δ 8.52 (s, 1H), 5.38 (bs, 2H). |
| 75% |
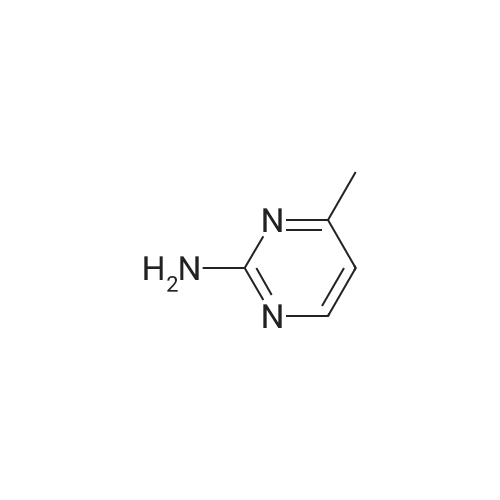
With N-Bromosuccinimide In chloroform at 20 - 50℃; for 20 h; |
4-(Trifluoromethyl)pyrimidin-2-ylamine (2 g, 12.3 mmol) was suspended in chloroform (70 ml) followed by the addition of N-bromosuccinimide (3.3 g, 18.4 mmol) and the resulting mixture was stirred at 50° C. for 5 hours and then at room temperature for 15 hours. A mixture of methylene chloride (50 ml) and 1 M sodium hydroxide (50 ml) was added, the resulting mixture was stirred, and then the organic layer was fractionated and dried over magnesium sulfate. The solvent was evaporated under reduced pressure to give the title compound (2.2 g, 75percent) as a pale orange solid.1H-NMR (CDCl3) δ: 5.37 (2H, brs), 8.52 (1H, s). |
| 73% |
With N-Bromosuccinimide In dichloromethane at 25℃; for 48 h; Darkness |
To a solution of compound 31(500mg, 3.06 mmol) in DCM (60 mL) was added NBS (1.66g, 9.32 mmol). The solution was stirred in the dark for 2d at rt. Then the reaction was quenched with iN NaOH (50 mL) and extracted with DCM (50 mL). The organic phase was washed with brine (100 mL), dried over Na2SO4, filtered and concentrated to give the title compound 32 as a white solid (530 mg, 73percent yield), which was used directly in the next step without further purification. ‘H NIVIR (CDC13): 8.52(s, 1H), 5.29(bs, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping