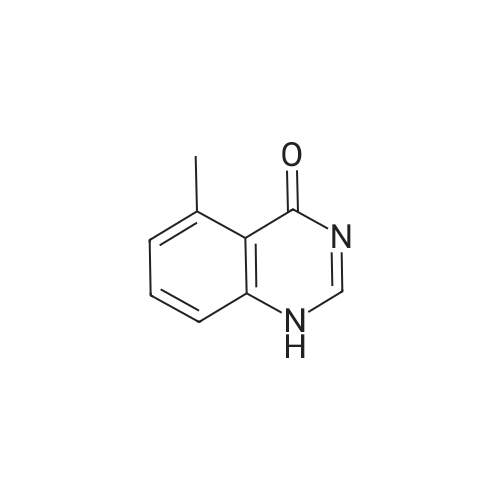
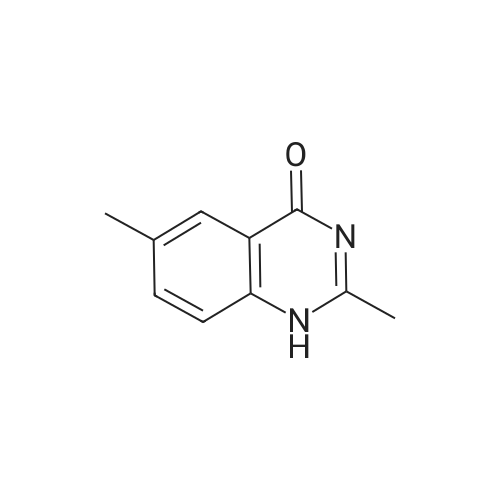
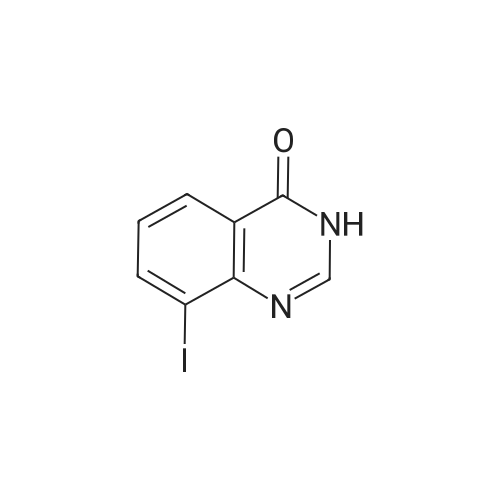
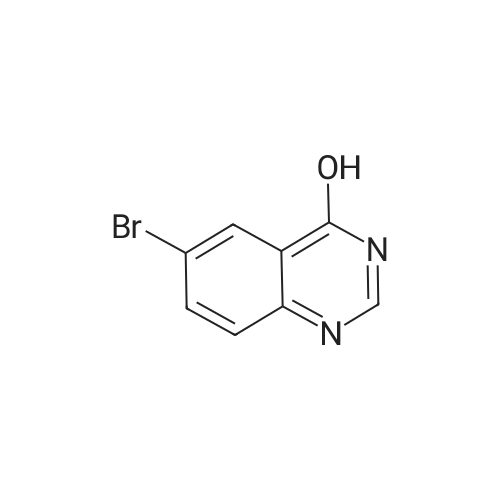
| 92.4% |
With thionyl chloride; In N,N-dimethyl-formamide; for 4h;Reflux; |
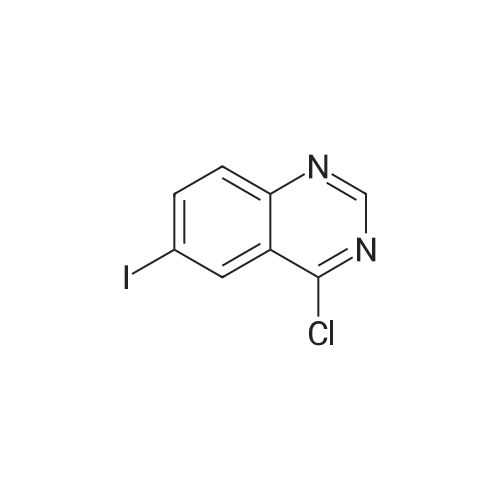
Compound 102 (14.4 g) was dissolved in thionyl chloride (50 ml)Adding 1 mL of the catalytic amount of anhydrous DMF,The reaction was refluxed for 4 hours.After completion of the reaction, excess thionyl chloride was distilled off under reduced pressure to give Compound 103. Yield 92.4%. |
| 88% |
With triethylamine; trichlorophosphate; In toluene; at 2 - 80℃; for 3.5h;Inert atmosphere; |
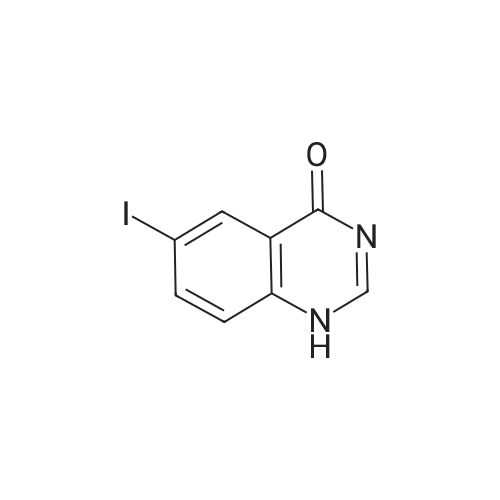
Solution of 6-iodo-quinazolin-4-one (0.54g, 2mmol) was added phosphorus oxychloride (0.37g, 2.4mmol), under nitrogen in dry toluene (2ml), triethylamine (0.24 g, 2.4mmol), leopard after the dropping was raised to 80 stirred 2.5h. The reaction solution was cooled to 2 stirred for 1h, and filtered. The filter cake was washed with acetone, washed with 1mol / L aqueous sodium hydroxide solution (in 3 ml of), washed with water and then with acetone. Vacuum dried to give a beige powder (0.5g, 88%) |
| 84% |
|
Oxalyl chloride (11.8 g, 8.1 mL, 92.6 mmol, 2.0 equiv) was added to a suspension of 6-iodoquinazolin-4-ol (12.6 g, 46.2 mmol, 1.0 equiv), DMF (0.5 ml) and 1,2-dichloroethane (300 mL) resulting in the reaction temperature increasing from 21 to 25 C. The mixture was heated at about 75 C. overnight. TLC (50% EtOAc/heptane) of an aliquot quenched with NaHCO3 showed the reaction to be incomplete. The mixture was cooled to room temperature, oxalyl chloride (2.0 mL, 0.5 equiv) added and the mixture refluxed for 7 hr. The clear dark brown solution was cooled to room temperature and poured very slowly into 10% aqueous Na2CO3 solution (500 mL). The aqueous mixture was extracted with EtOAc (500 mL). Most of the aqueous phase was separated and the remaining mixture filtered to remove some insolubles at the interface. The phases of filtrate were separated, the organic phase washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The resulting solid was triturated with cold heptane (about 200 mL), filtered and dried to give 11.2 g (84%) of 14 as a light brown solid that was used without further purification. |
| 77% |
With thionyl chloride; In N,N-dimethyl-formamide; for 6h;Reflux; |
4-chloro-6-iodoquinazoline 6-iodo-4(1H)-quinazolinone (10 g, 37 mmol) was weighed into a 250 mL flask. Thionyl chloride (100 mL, 1.4 mmol) and DMF (0.5 mL, 6.5 mmol) were added to give a grey suspension. The mixture was heated to reflux. Heating was continued for 6 h and then the mixture was cooled on ice bath for 1 h. A yellow solid precipitated and was collected by filtration to afford 8.6 g (77%) of the title compound. |
| 71% |
With N-ethyl-N,N-diisopropylamine; trichlorophosphate; at 0 - 90℃; |
Step B: Preparation of 4-chloro-6-iodoquinazoline: To a cooled (0 C) suspension of 6-iodoquinazolin-4-ol (107.6 g, 396 mmol) and DIEA (138 mL, 791 mmol) in dichloroethane (600 mL) was added POCl3 (44.25 mL, 475 mmol). After heating to 90 C for 16 hours, the reaction mixture was cooled to room temperature and the crystals (73.8 g) collected by filtration. The filtrate was concentrated under reduced pressure and azeotroped twice with toluene. The solids (8.3 g) were triturated with isopropanol (450 mL) and cooled in an ice bath before collecting by filtration and drying under high vacuum. The two solids were combined to provide the product (82.1 g, 71%) as white solid. |
|
With oxalyl dichloride; N,N-dimethyl-formamide; In 1,2-dichloro-ethane; at 0 - 110℃; for 1.5h;Heating / reflux; |
EXAMPLE 11; 4-(6-Iodo-quinazolin-4-yl)-piperidine-1-carboxylic acid (4-isopropoxy-phenyl)-amide; a. 4-Chloro-6-iodo-quinazoline; A mixture of 5-iodoanthranilic acid (9.96 g, 37.9 mmol) and formamidine acetate (4.20 g, 40.3 mmol) (adapted from J. Org. Chem. 51:616, 1986) in absolute EtOH (80 mL) was refluxed under air for 2 h. The smoky amber solution with heavy white precipitate was then concentrated under reduced pressure at 90 C., and residual protic solvent was removed with toluene rotary evaporation (2×100 mL) at 90 C. The resulting sticky tan solid was treated with a thick white slurry of Vilsmeier-Haack reagent in one portion under air at rt. [The Vilsmeier-Haack reagent was prepared by the addition of a solution of oxalyl chloride (10.9 mL, 125 mmol) in DCE (44 mL) to a solution of DMF (6.7 mL, 87 mmol) in DCE (21 mL) dropwise over 10 min at 0 C. with vigorous stirring. The ice bath was removed immediately following completion of oxalyl chloride addition, and the white slurry was stirred at “rt” for 5 min before transfer to the crude 4-hydroxy-6-iodo-quinazoline intermediate.] The reaction was then refluxed under air (oil bath 110 C.) for 1 h 15 min, and the resulting homogeneous brown solution was allowed to cool to rt, at which point a heavy precipitate formed. The reaction was poured into ice water (300 mL) and extracted with DCM (3×250 mL). The opaque organic layers were combined, dried (Na2SO4), and filtered to provide a clear red amber filtrate. Concentration under reduced pressure, followed by toluene rotary evaporation at 90 C. to remove potentially reactive volatiles, afforded the title compound as a tan powder (8.41 g, 94% from iodoanthranilic acid) suitable for treatment with LiHMDS in the next step. 1H-NMR (300 MHz, CDCl3) δ 9.07 (s, 1H), 8.67 (dd, 1H), 8.22 (dd, 1H), 7.81 (d, 1H). |
|
With thionyl chloride; In N,N-dimethyl-formamide; at 80℃; for 5h;Reflux; |
6-chloro-4-hydroxyquinazoline (2.72 g, 10 mmol) and DMF (0.2 mL) were added to 24 mL of dichlorosulfoxide and refluxed5h. The thionyl chloride was distilled off under reduced pressure, and the remaining thionyl chloride was removed by steaming with ethyl acetate (100 mL x 3) to give a yellow solid 4-chloro-6-iodoquinazoline. 20 mL of isopropanol, 3-bromoaniline (2.06 g, 12 mmol) and triethylamine (1.00 g, 10 mmol) and refluxed at 80 C for 6 h. Cooled, filtered, the filtrate added ether to continue to precipitate the solid, the resulting solid with a large number of water and then Followed by suction filtration, drying, pale yellow solid 3.61g, yield 84.6%, Mp: 197-199 . |
|
With triethylamine; trichlorophosphate; at 90℃; for 6h;Inert atmosphere; |
At 0 C,The compound 6-iodo-4-quinazolinone (10 g, 30 mmol) was added to toluene (150 mL) under N2.Add triethylamine (9.09 g, 90 mmol),Phosphorus oxychloride (6.12 g, 40 mmol) was slowly added to the above solution, and a large amount of white smoke appeared, and the temperature was slowly raised to 90 C to stir the reaction for 6.0 h.Toluene (200 mL) was added to the above solution.The organic phase was washed once with saturated brine (100 mL).Anhydrous sodium sulfate (20 g) was dried and concentrated to give a red brown oil.This crude product was used in the next reaction without purification. |
|
With thionyl chloride; In N,N-dimethyl-formamide; at 25 - 90℃; for 3h;Inert atmosphere; |
To a stirred solution of 6-iodoquinazolin-4-ol (500 mg, 1.84 mmol) in thionyl chloride (7 mL)at 25C under nitrogen was added DMF (0.2 mL) and the reaction mixture was heated to 90Cfor 3 hours. The resulting mixture was evaporated to dryness and the residue was azeotropedwith excess toluene to afford 4-chloro-6-iodoquinazoline which was used without furtherpurification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping