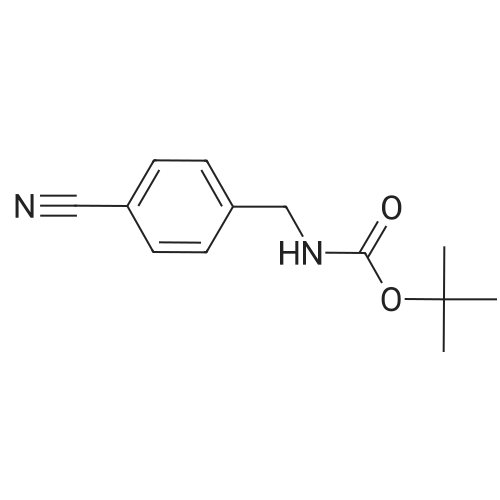
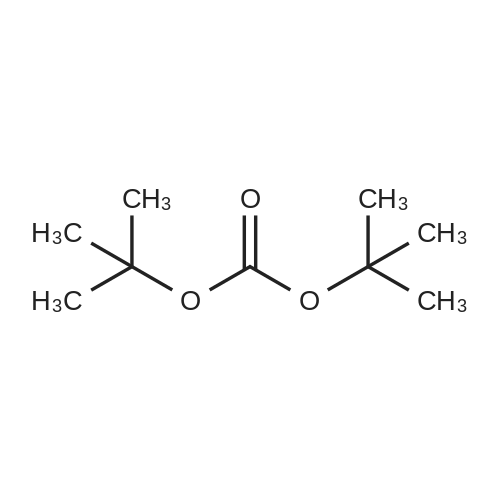
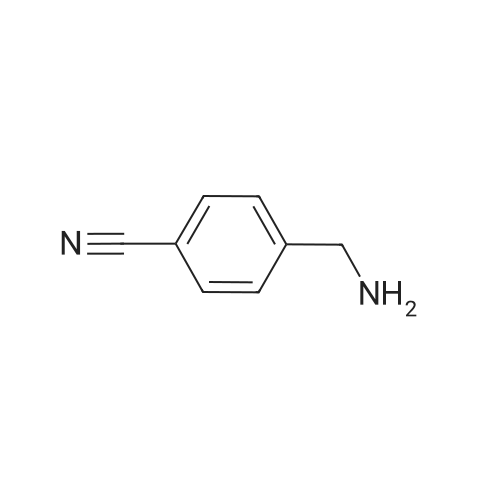
| 94% |
With sodium carbonate; In dichloromethane; at 0 - 20℃; |
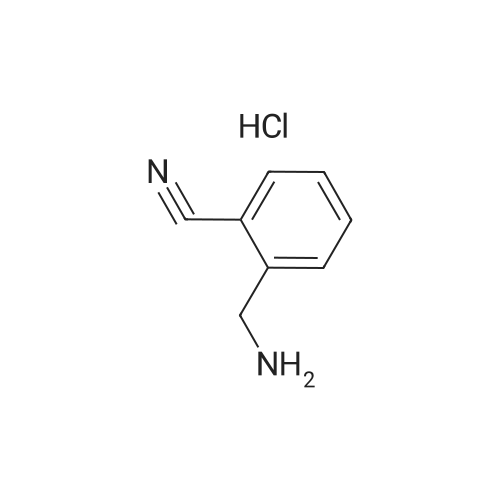
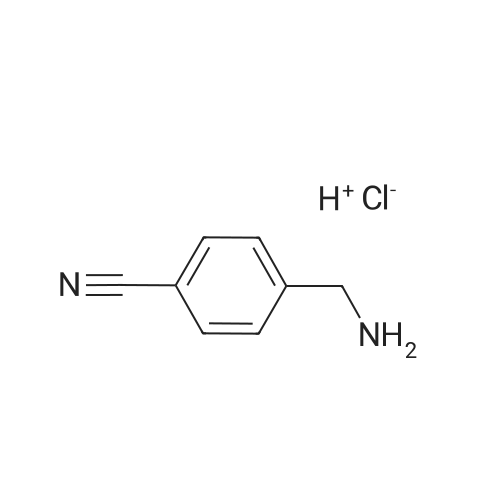
To a solution of 4-aminomethylbenzonitrile hydrochloride (n-1) (5.00 g, 29.7 mmol) in dichloromethane(167 mL), sodium carbonate (7.54 g, 71.2 mmol) was added. The reaction mixture was cooled to 0C and di-tert-butyl dicarbonate (7.57 mL, 32.6 mmol) was added; the resulting mixture was brought to room temperature at which it was stirred overnight. To the reaction mixture, water was added and extraction was conducted with ethyl acetate. The organic layer was successively washed with water and saturated brine and dried over anhydrous sodium sulfate. The solvents were distilled off under reduced pressure and the residue was purified by silica gel (NH) column chromatography (n-hexane:ethyl acetate = 1:1) to give tert-butyl 4-cyanobenzylcarbamate (n-2) (amount, 6.48 g; yield, 94%). |
| 80% |
With sodium hydroxide; In water; for 16h; |
Intermediate 28: tert-butyl {4-[amino(hvdroxyirnino)methyllbenzyl}carbamateStep 1: tert-butyl 4-cyanobenzylcarbamate To a solution of 4-cyanobenzylamine hydrochloride (1.05 g; 6.25 mmol) in water (10 mL) was added sodium hydroxide (0.75 g; 18.75 mmol) and di-tert-butyldicarbonate (1 .49 g; 6.87 <n="114"/>mmol) and the mixture was stirred for 16 hours. The solid was collected by filtration and dried in a vacuum oven at 40C for 48 hours. The title compound was isolated as a white solid (1 .35 g; 80%). 1H NMR: (CDCI3, 400MHz) delta 7.62 (2H, d, J = 8.1 Hz), 7.39 (2H, d, J = 8.0 Hz), 4.97 (1 H, s), 4.37 (2H, d, J = 6.2 Hz), 1.46 (9H, s). |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0℃; for 4.16667h; |
100 g (0.593 mol) of 4-cyanobenzylamine×HCl were dissolved in 1.2 l of dioxane and 600 ml of 2 N NaOH. 142.3 g (0.652 mol) of di(tert-butyl)pyrocarbonates were added in two portions over 10 min at 0 C. The pH was adjusted to 9-10 by adding 2 N NaOH, and the mixture was stirred for a further 4 h. The solvent was removed in vacuo, and the residue was taken up with ethyl acetate, washed 3× each with 5% KHSO4 and NaCl-saturated water and then dried over Na2SO4. The solvent was removed in vacuo (white solid). Yield: 132.6 g (0.57 mol) of white solid, HPLC: 51.6% B |
|
With triethylamine; In dichloromethane; |
Synthesis of tert-butyl(4-cyanobenzyl)carbamate (7) (0149) 4-Cyanobenzylamine HCl (5.00 g, 29.7 mmol, 1.0 eq) was suspended in CH2Cl2 and NEt3 (10.3 mL, 74.1 mmol, 2.5 eq) were added to obtain a clear solution. Boc2O (8.86 mL, 38.6 mmol, 1.3 eq) was added and the solution was stirred overnight. The reaction mixture was diluted to a total volume of 100 mL with CH2Cl2 and washed with 1 M HCl (3×15 mL) and sat. NaHCO3 (3×15 mL). The organic layer was dried over MgSO4 and concentrated under reduced pressure. (0150) 1H NMR (500 MHz, CDCl3) delta 7.58-7.52 (m, 2H), 7.35-7.29 (m, 2H), 4.88 (s, 1H), 4.30 (d, J=6.3 Hz, 2H), 1.39 (s, 9H). (0151) 13C NMR (126 MHz, CDCl3) delta 156.2, 145.0, 132.8, 128.2, 119.1, 111.6, 80.5, 44.6, 28.8. (0152) HRMS (ESI): m/z calc. for C13H16N2NaO2+: 255.1104. found: 255.1110 (Delta=-2.0 ppm). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping