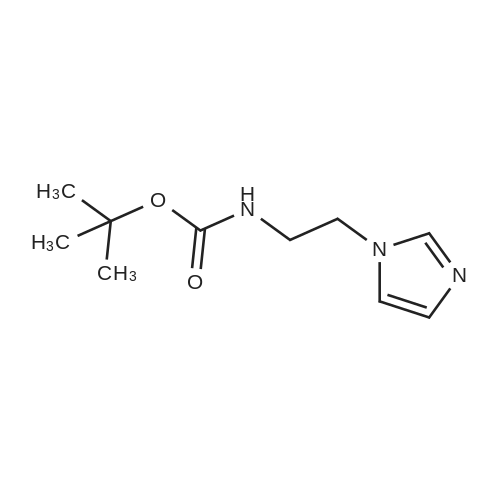
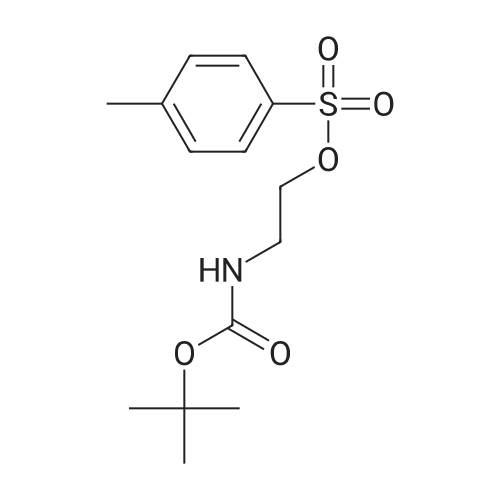
| 29% |
|
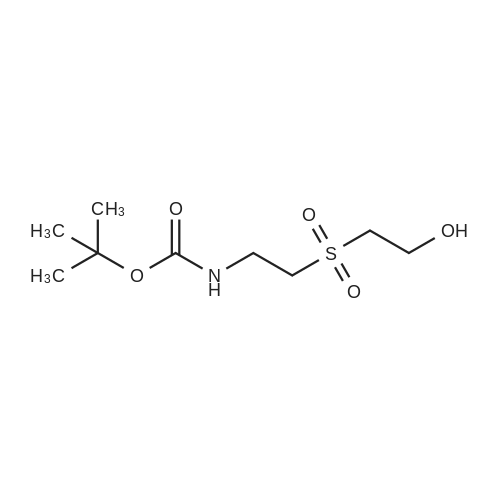
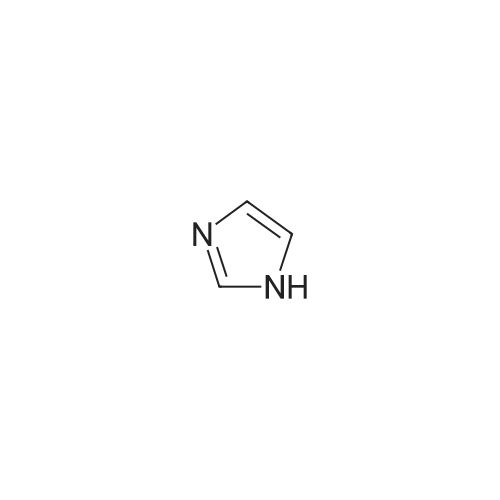
[0199] Tosyl chloride (1.50 g, 7.87 mmol) was added to a solution of (2-hydroxy-ethyl)-carbamic acid tert-butyl ester (0.84 g, 5.2 mmol) and Et3N (1.23 mL, 8.82 mmol) in CH2Cl2 (26 mL), and the solution was stirred at room temperature for 17 h. The solution was washed with H2O (15 mL), and the aqueous phase was extracted with CH2Cl2 (10 mL). The combined organic phases were dried (MgSO4) and concentrated in vacuo. Purification of the crude material by column chromatography on silica gel (20% EtOAc/hexanes) gave yellow crystals (1.29 g, 79%). 1H NMR (CDCl3) δ 1.41 (s, 9H), 2.45 (s, 3H), 3.38 (m, 2H), 4.07 (m, 2H), 4.82 (br s, 1H), 7.35 (d, 2H, J=8.1 Hz), 7.79 (d, 2H, J=8.1 Hz). [0200] (2-Imidazol-1-yl-ethyl)-carbamic Acid Tert-Butyl Ester. [CHEMMOL-00028] [0201] A solution of imidazole (253 mg, 3.72 mmol) in DMF (2 mL) was added to a suspension of NaH (60% in mineral oil, 164 mg, 4.10 mmol) in DMF (8 mL), and the mixture was stirred at room temperature for 45 minutes. A solution of <strong>[158690-56-3]toluene-4-sulfonic acid 2-tert-butoxycarbonylamino-ethyl ester</strong> (1.29 g, 4.09 mmol) in DMF (6 mL) was added, and the mixture was stirred at room temperature for 16 h then concentrated in vacuo. The residue was partitioned between H2O (25 mL) and EtOAc (25 mL), and the aqueous phase was extracted with EtOAc (25 mL). The combined organic phases were dried (MgSO4) and concentrated in vacuo. Purification of the crude material by column chromatography on silica gel (200:5:1-100:5:1 CH2Cl2/MeOH/NH4OH) gave a colourless oil (224 mg, 29%). 1H NMR (CDCl3) δ 1.44 (s, 9H), 3.43 (m, 2H), 4.08 (m, 2H), 4.64 (br s, 1H), 6.92 (s, 1H), 7.09 (s, 11H), 7.46 (s, 1H). [0202] (2-Imidazol-1-yl-ethyl)-(5,6,7,8-tetrahydro-quinolin-8-yl)-amine. [CHEMMOL-00029] [0203] A solution of (2-imidazol-1-yl-ethyl)-carbamic acid tert-butyl ester (224 mg, 1.06 mmol) in 1:1 TFA/CH2Cl2 (4 mL) was stirred at room temperature for 1 h then concentrated in vacuo. The residue was dissolved in 1 N NaOH(aq) (10 mL) then saturated with sodium chloride and extracted with CHCl3 (5×15 mL). The combined organic extracts were dried (MgSO4) and concentrated in vacuo to give a yellow oil (55 mg). [0204] Using General Procedure B: To a stirred solution of the amine from above (55 mg), 6,7-dihydro-5H-quinolin-8-one (73 mg, 0.50 mmol), and AcOH (0.030 mL, 0.52 mmol) in THF (5 mL) was added NaBH(OAc)3 (315 mg, 1.49 mmol) and the mixture was stirred at room temperature for 2 h. The crude material was dissolved in saturated HBr/AcOH (2 mL) and stirred at room temperature for 15 minutes. The solution was made basic with 10 N NaOH(aq) and extracted with CH2Cl2 (3×15 mL). The combined organic extracts were dried (MgSO4) and concentrated in vacuo. Purification of the crude material by column chromatography on silica gel (200:5:1 CH2Cl21MeOH/NH4OH) gave a yellow oil (92 mg, 77%). 1H NMR (CDCl3) δ 1.73 (m, 2H), 1.91-2.13 (m, 2H), 2.76 (m, 2H), 3.12 (m, 2H), 3.78 (m, 1H), 4.11 (m, 2H), 7.01 (s, 1H), 7.08 (m, 2H), 7.38 (d, 1H, J=7.5 Hz), 7.56 (s, 1H), 8.37 (d, 1H, J=3.9 Hz). [0205] 2-[(2-Imidazol-1-yl-ethyl)-(5,6,7,8-tetrahydro-quinolin-8-yl)-amino]-methyl}-benzimidazole-1-carboxylic Acid Tert-Butyl Ester. [CHEMMOL-00030] [0206] A mixture of (2-imidazol-1-yl-ethyl)-(5,6,7,8-tetrahydro-quinolin-8-yl)-amine (92 mg, 0.37 mmol), 2-chloromethyl-benzimidazole-1-carboxylic acid tert-butyl ester (101 mg, 0.379 mmol), potassium iodide (3 mg, 0.02 mmol), and N,N-diisopropylethylamine (0.10 mL, 0.57 mmol) in acetonitrile (4 mL) was heated at 60 C. for 15 h. Saturated NaHCO3 (aq) (15 mL) was added, and the mixture was extracted with CH2Cl2 (3×15 mL). The combined organic extracts were dried (MgSO4) and concentrated in vacuo. Purification of the crude material by column chromatography on silica gel (250:5:1 CH2Cl2/MeOH/NH4OH) gave a yellow oil (21 mg, 12%). 1H NMR (CDCl3) δ 1.45 (m, 1H), 1.66 (m, 10H), 1.91 (m, 2H), 2.69 (m, 2H), 2.92 (m, 1H), 3.18 (m, 1H), 3.67 (m, 2H), 4.20 (dd, 1H, J=10, 5.6 Hz), 4.67 (d, 1H, J=15 Hz), 4.80 (d, 1H, J=15 Hz), 6.74 (s, 1H), 6.90 (s, 1H), 7.01 (dd, 1H, J=7.7, 4.7 Hz), 7.33 (m, 4H), 7.73 (m, 1H), 7.86 (m, 1H), 8.38 (d, 1H, J=3.3 Hz). [0207] (1H-Benzimidazol-2-ylmethyl)-(2-imidazol-1-yl-ethyl)-(5,6,78-tetrahydro-quinolin-8-yl)-amine (COMPOUND 12). [0208] A solution of 2-[(2-imidazol-1-yl-ethyl)-(5,6,7,8-tetrahydro-quinolin-8-yl)-amino]-methyl}-benzimidazole-1-carboxylic acid tert-butyl ester (21 mg, 0.044 mmol) in 3:1 TFA/CH2Cl2 (4 mL) was stirred at room temperature for 30 minutes then concentrated in vacuo. The residue was partitioned between CH2Cl2 (20 mL) and 1 N NaOH(aq) (10 mL), and the aqueous phase was extracted with CH2Cl2 (10 mL). The combined organic extracts were dried (MgSO4) and concentrated in vacuo to afford COMPOUND 12 as a yellow foam (15 mg, 83%). 1H NMR (CDCl3) δ 1.73 (m, 2H), 1.99 (m, 1H), 2.20 (m, 1H), 2.69-2.88 (m, 2H), 2.92-3.08 (m, 2H), 3.82-3.98 (m, 2H), 4.04 (d, 1H, J=17 Hz), 4.09 (m, 1H), 4.19 (d, 1H, J=17 Hz), 6.70 (s, 1H), 6.93 (s, 1H), 7.18 (m, 3H... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping