| 85.8% |
Stage #1: With sodium methylate In methanolInert atmosphere
Stage #2: at 0 - 5℃; Inert atmosphere
Stage #3: With hydrogenchloride In methanol; water at 20 - 40℃; Inert atmosphere |
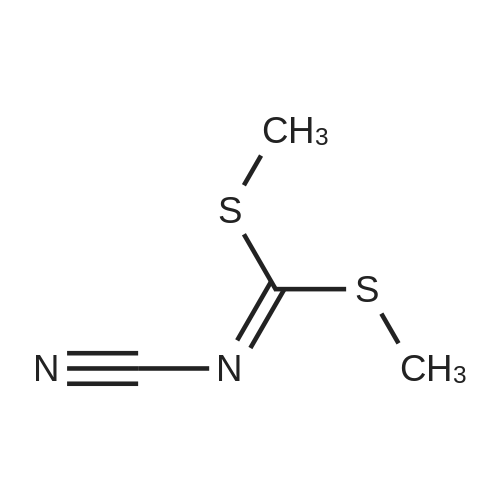
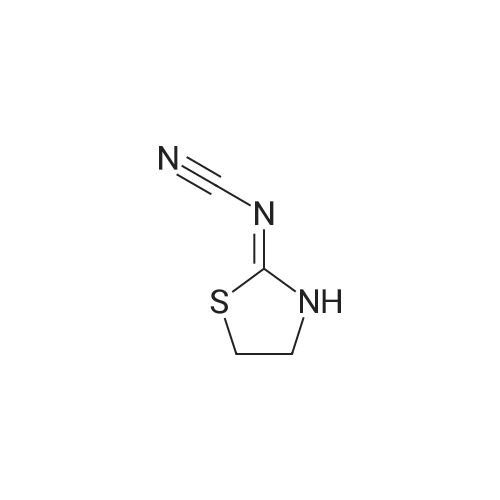
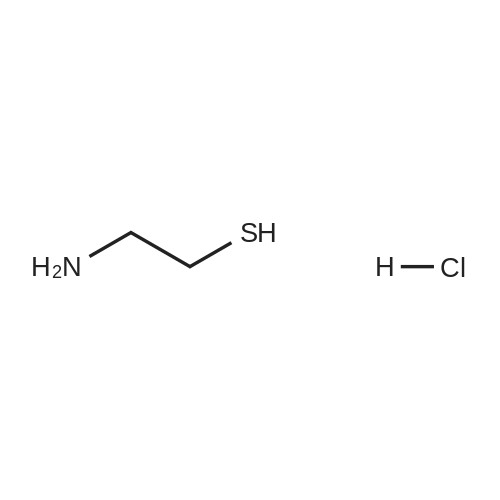
EXAMPLE 3; A 1 litre four-necked flask provided with a thermometer and stirrer is charged with 9Og of methanol (2.815 moles) and 36g of sodium methoxide (0.667 moles, 1.3 mole equivalent based upon 1.1 mole equivalent of 2-aminoethane thiol hydrochloride) under nitrogen. The mixture is cooled and stirred to dissolve in <n="16"/>methanol, then 63.5g of 2-aminoethane thiol hydrochloride (0.564 moles) is added thereto and dissolved therein. The reaction mixture is cooled to 0° C and 75g of dimethyl N-cyanoiminodithiocarbonate (0.514 moles) is added keeping the inside temperature at 5° C or less. After the end of addition, the mixture is allowed to react at 0 to 5° C for 2 hours under nitrogen. Thereafter, the reaction mixture is heated to 20° C and pH is adjusted to 4 with aqueous hydrochloric acid solution (36percent w/w), then further heated to 40° C and stirred for 2 hours. After stirring, the reaction mixture is cooled to 0° C and the crystals are suction filtered and the slurry thus obtained is washed with 225ml of chilled water to obtain 66g of 2-cyanoimino-l, 3-thiazolidine. The wet crystals are dried in vacuo at 80° C under reduced pressure for 5 hours to obtain 58g of 2-cyanoimino-l, 3-thiazolidine (yield 85.8percent) with 99.9percent HPLC purity. |
| 81.6% |
Stage #1: With sodium hydroxide In water
Stage #2: for 2 h;
Stage #3: With hydrogenchloride In water at 20 - 40℃; for 2 h; |
Example 1
A 300 ml four-necked flask provided with a thermometer and an agitator was charged with 150 g of water (8.3 moles), then 11.2 g of 99percent by weight sodium hydroxide (0.28 mole, 1.12 moles based upon 1 mole of 2-aminoethane thiol hydrochloride) was added while cooling and agitating to be dissolved in the water, then 28.8 g of 99.5percent by weight 2-aminoethane thiol hydrochloride (0.25 mole) was added thereto and dissolved therein, then the reaction mixture was cooled to 0°C.
To this reaction mixture, 36.9 g of 99.5percent by weight dimethyl N-cyanoiminodithiocarbonate ester (0.25 mole) was added so that the temperature inside the system became 5°C or less..
After the end of addition, the mixture was allowed to react at 0 to 5°C for 2 hours.
Thereafter, the reaction mixture was heated to 20°C, adjusted in PH to 4.0 with 36percent by weight aqueous hydrochloric acid solution, then further heated to 40°C and allowed to age for 2 hours.
After aging, the reaction mixture was cooled to 20°C, then the crystals were suction filtered and washed with 100 g of water (5.6 moles) to obtain 36.6 g of 2-cyanoimino-1,3-thiazolidine.
The wet crystals were dried in vacuo at 84°C under 6.7 x 10-4 MPa for 5 hours to obtain 28.6 g of 99.7percent purity 2-cyanoimino-1,3-thiazolidine (yield 89.8percent = value converted to purity, based on the charged dimethyl N-cyanoiminodithiocarbonate ester). Note that the purity was analyzed by means of high pressure liquid chromatography (HPLC). Example 2; A 300 ml four-necked flask provided with a thermometer and an agitator was charged with 150 g of water (8.3 moles), then 12.3 g of 99percent by weight sodium hydroxide (0.31 mole, 1.24 moles based upon 1 mole of 2-aminoethane thiol hydrochloride) was added, while cooling and agitating to be dissolved in the water, then 28.8 g of 99.5percent by weight 2-aminoethane thiol hydrochloride (0.25 mole) was added thereto and dissolved therein. Thereafter, the reaction mixture was cooled to 0°C., then 36.9 g of 99.5percent by weight dimethyl N-cyanoiminodithiocarbonate ester (0.25 mole) was gradually added so that the temperature inside the system became 5°C or less. After the end of addition, the mixture was allowed to react for further 2 hours. Next, the reaction mixture was heated to 20°C, adjusted in pH to 3.9 with 4.56 g (0.045 mole) of 36percent by weight aqueous hydrochloric acid solution, then further heated to 40°C and allowed to age for 2 hours. After aging, the reaction mixture was cooled to 20°C, then suction filtered and washed with 200 g of water (11.1 moles) to obtain 34.7 g of wet crystals of 2-cyanoimino-1,3-thiazolidine. The wet crystals obtained above, were dried in vacuo at 80°C under 6.7 x 10-4 MPa for 5 hours to obtain 27.9 g of 100percent purity 2-cyanoimino-1,3-thiazolidine (yield 87.9percent = value converted to purity, based on charged dimethyl N-cyanoiminodithiocarbonate ester). Further, the total weight of the filtrate combined with the washings was 371.5 g. The filtrate included 0.98percent of 2-cyanoiminothiazolidine (value converted to yield: 11.5percent). Example 3; A 300 ml four-necked flask provided with a thermometer and an agitator was charged with 150 g of water (8.3 moles), then 10.6 g of 99percent by weight sodium hydroxide (0.26 mole, 1.04 moles based upon 1 mole of 2-aminoethane thiol hydrochloride) was added, while cooling and agitating to be dissolved in the water, then 28.8 g of 99.5percent by weight 2-aminoethane thiol hydrochloride (0.25 mole) was added thereto and dissolved therein. Thereafter, the reaction mixture was cooled to 0°C, then 36.9 g of 99.5percent by weight dimethyl N-cyanoiminodithiocarbonate ester (0.25 mole) was gradually added so that the temperature inside the system became 5°C or less, then was allowed to react for 2 hours. Next, the reaction mixture was heated to 20°C, adjusted in pH to 9.2 with 0.25 g of 36percent by weight aqueous hydrochloric acid solution (0.0025 mole), then further heated to 40°C and allowed to age for 2 hours. After aging, the reaction mixture was cooled to 20°C, then suction filtered and washed with 200 g of water (11.1 moles) to obtain 36.33 g of wet crystals of 2-cyanoimino-1,3-thiazolidine. The wet crystals were dried in vacuo at 80°C under 6.7 x 10-4 MPa for 5 hours to obtain 27.2 g of 95.2percent purity 2-cyanoimino-1,3-thiazolidine (yield 81.6percent = value converted to purity, based on the charged dimethyl N-cyanoiminodithiocarbonate ester). Further, the total weight of the filtrate combined with the washings was 361.5 g. The filtrate included 0.95percent of 2-cyanoiminothiazolidine (value converted to yield: 10.8percent). |
| 59.4% |
Stage #1: With sodium hydroxide In water
Stage #2: for 2 h;
Stage #3: With hydrogenchloride In water |
Comparative Example 2
A 300 ml four-necked flask provided with a thermometer and an agitator was charged with 114 g of water (6.3 moles), then 20.9 g of 99percent by weight sodium hydroxide (0.50 mole) was added, while cooling and agitating, to be dissolved therein, then 28.8 g of 99.5percent by weight 2-aminoethane thiol hydrochloride (0.25 mole) was added thereto and dissolved therein..
Then, the reaction mixture was cooled to 0°C, then 36.9 g of 99.5percent by weight dimethyl N-cyanoiminodithiocarbonate ester (0.25 mole) was gradually added, while holding the temperature to 5°C or less, then the mixture was allowed to react for 2 hours.
Next, 30.9 g of 36percent by weight aqueous hydrochloric acid solution (0.29 mole) was added to try to adjust the PH, but the mixture violently started bubbling at around PH 9.0 and finally the PH became 9.5..
Next, the reaction mixture was suction filtered and washed with 200 g of water (11.1 moles) to obtain 28.6 g of wet crystals of 2-cyanoimino-1,3-thiazolidine.
The wet crystals were dried in vacuo at 80°C under 6.7 x 10-4 MPa for 5 hours to obtain 18.9 g of 100percent purity 2-cyanoimino-1,3-thiazolidine (yield 59.4percent = value converted to purity, based on the charged dimethyl N-cyanoiminodithiocarbonate ester). Comparative Example 3; A 300 ml four-necked flask provided with a thermometer and an agitator was charged with 150 g of water (8.3 moles), then 20.9 g of 99percent by weight sodium hydroxide (0.50 mole) was added, while cooling and agitating, to be dissolved, then 28.8 g of 99.5percent by weight 2-aminoethane thiol hydrochloride (0.25 mole) was added thereto and dissolved therein. Thereafter, the reaction mixture was cooled to 0°C, then 36.9 g of 99.5percent by weight dimethyl N-cyanoiminodithiocarbonate ester (0.25 mole) was gradually added, while holding the temperature to 5°C or less, the mixture was then allowed to react for 2 hours. Next, 31.7 g of 36percent by weight aqueous hydrochloric acid solution (0.31 mole) was added to try to adjust the pH, but the mixture violently started bubbling at around pH 9.0 and finally the pH became 1.7. Next, the reaction mixture was suction filtered and washed with 200 g of water (11.1 moles) to obtain 34.8 g of wet crystals of 2-cyanoimino-1,3-thiazolidine. The wet crystals were dried in vacuo at 80°C under 6.7 x 10-4 MPa for 5 hours to obtain 20.7 g of 100percent purity 2-cyanoimino-1,3-thiazolidine (yield 65.2percent = value converted to purity, based on the charged dimethyl N-cyanoiminodithiocarbonate ester). |
| 47.7% |
Stage #1: With sodium hydroxide In water
Stage #2: for 3 h; Heating / reflux |
Comparative Example 1
A 300 ml four-necked flask provided with a thermometer and an agitator was charged with 100 g of water (5.6 moles), then 10.1 g of 99percent by weight sodium hydroxide (0.25 mole) was added, while cooling and agitating to dissolve it..
Next, 28.8 g of 99.5percent by weight 2-aminoethane thiol hydrochloride (0.25 mole) was added thereto and dissolved therein, then 36.9 g of dimethyl N-cyanoiminodithiocarbonate ester (0.25 mole) was added and the mixture was heated and refluxed under agitation for 3 hours..
After the end of the reaction, the reaction mixture was cooled to room temperature, then the crystals were suction filtered and washed with methanol and dried in vacuo at 80°C under 6.7 x 10-4MPa for 5 hours to obtain 15.2 g of 99.7percent purity 2-cyanoimino-1,3-thiazolidine (yield 47.7percent = value converted to purity, based on the charged dimethyl N-cyanoiminodithiocarbonate ester).
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping