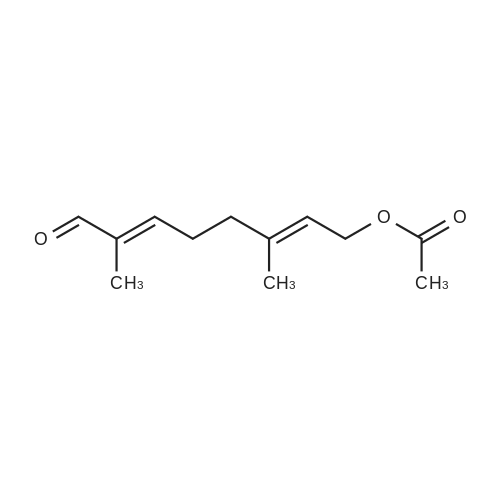
Alternatived Products of [ 1530-33-2 ]

Product Citations
Investigating the Mechanism and Expanding the Reactivity of Cu (I)-Catalyzed [2+ 2] Photocycloaddition Reactions of Olefins
Gethmini Kasunka Jayasekara
;
University of Rhode Island,2023.
More
Abstract: Light-matter interactions can change the physical and chemical properties of a substance. The energy of photons can be used to dissociate the chemical bonds as well as to generate new bonds. The work in this dissertation is an attempt to understand how photon energy governs the formation of new carbon bonds via alkene groups in the presence of copper catalyst.
Intermolecular and intramolecular [2+2] photocycloaddition reactions facilitate the generation of cyclobutane moieties which are otherwise difficult to synthesize. Transition metal-based salts are used as catalysts to promote photocycloaddition reactions. The structure and the ring strain of a substrate can affect the reactivity and the rate of the photocycloaddition reactions. In this
dissertation chapter 2 focuses on understanding the mechanism of Copper(I)-catalyzed intermolecular [2+2] photocycloaddition reactions. We have used norbornene and cyclohexene as model substrates to explicate the mechanism of the Cu(I)-catalyzed intermolecular photocycloaddition reactions by using Xray and optical transient absorption techniques. The mechanism for norbornene shows an initial metal to ligand charge transfer (MLCT) state which lasts for 18ns prior to reverse electron transfer. Cyclohexene shows a change in metalligand bond strength instead of undergoing charge transfer. The evidence of the change in bond strength is consistent with the proposed idea of Salomon and Kochi for the formation of a more strained trans-cyclohexene intermediate.
The work presented in Chapter 3 of this dissertation focuses on understanding the mechanism of Cu(I)-catalyzed intramolecular [2+2] photocycloaddition reactions through the utilization of experimental and theoretical methods. The model substrates used for this study are 1,6-heptadiene, 1,7-octadiene, and 1,8-nonadiene which are linear alkenes with different carbon chain lengths. According to literature, only 1,6-dienes have been shown to undergo Cu(I)-catalyzed [2+2] photocycloaddition reactions. Theoretical DFT/TD-DFT calculations and experimental high energy resolution X-ray absorption spectroscopic studies are done to understand the structurefunctional differences in photoreactive and nonreactive substrates. Based on the correlations observed between structure and photoreactivity, we have modified the structure of non-reactive substrates to adopt photoreactive conformations with steric modifications. Nuclear Magnetic Resonance (NMR) spectroscopy is used to confirm the photoreactivity of the alkene substrates.
Purchased from AmBeed:
1530-33-2
-Catalyzed [2+ 2] Photocycloaddition Reactions of Olefins.png)
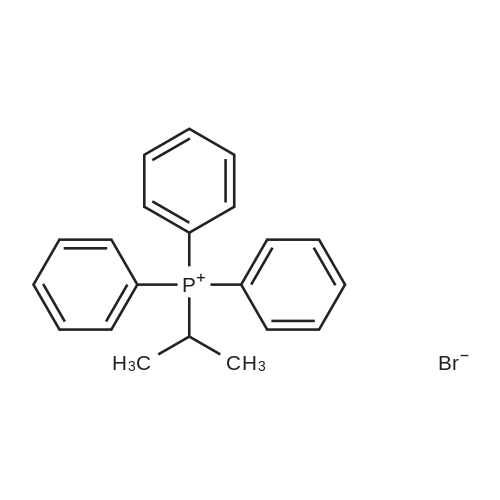
Product Details of [ 1530-33-2 ]
| CAS No. : | 1530-33-2 |
MDL No. : | MFCD00064807 |
| Formula : |
C21H22BrP
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | HSOZCYIMJQTYEX-UHFFFAOYSA-M |
| M.W : |
385.28
|
Pubchem ID : | 2795292 |
| Synonyms : |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


-Catalyzed [2+ 2] Photocycloaddition Reactions of Olefins.png)