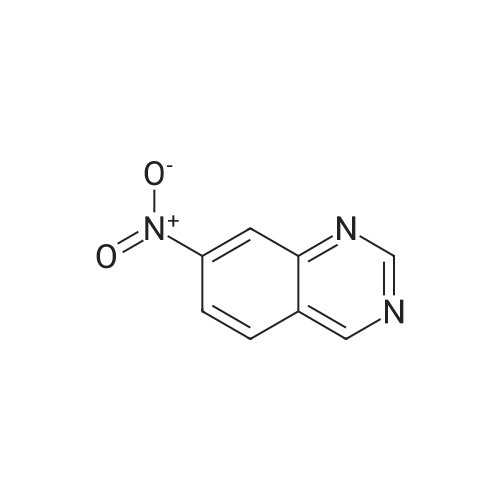
| 79% |
With copper(l) iodide; N,N`-dimethylethylenediamine; In 1,4-dioxane; at 120℃; for 0.5h;Microwave irradiation; |
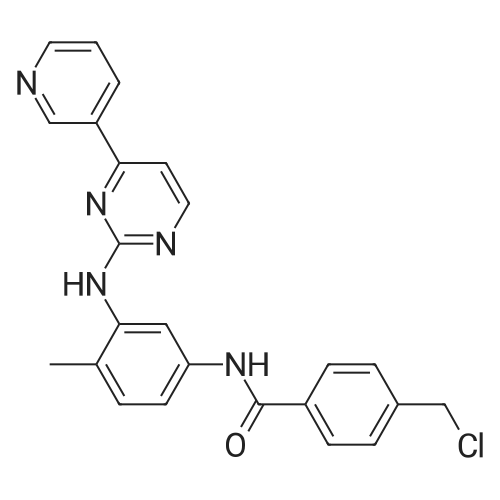
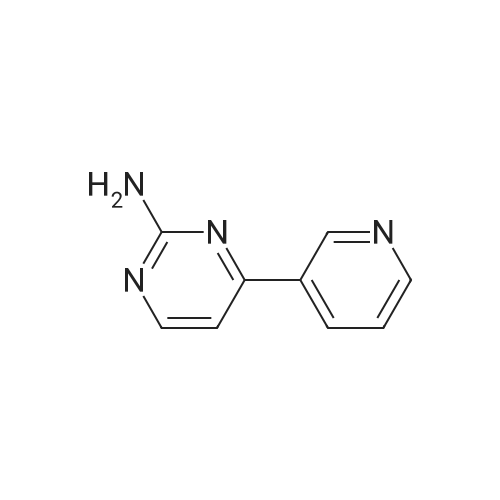
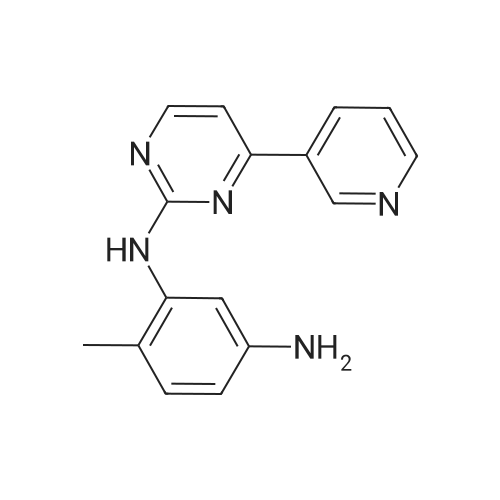
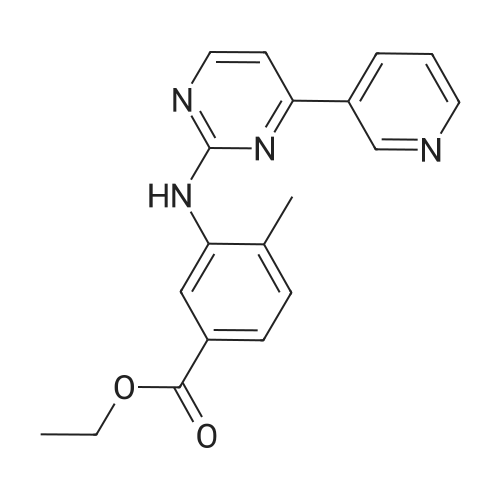
The mixture of 4-(Pyridin-3-yl) pyrimidin-2- amine (0.378 g,2.2 mmol), copper iodide (0.5 mmol) and anhydrous K2CO3(4 mmol), Dioxane (5 mL), 2-bromo-1-methyl-4- nitrobenzene(2 mmol), and DMEDA (0.5 mmol) was irradiated in the microwave synthesizer and heated at 120 C for 30 min. After completion of the reaction, ammonium hydroxide and brine solution was added andthen extracted with ethyl acetate. The organic layer was dried overanhydrous sodium sulphate, the solvent was evaporated in buchirotary evaporator and the resulting solid was dried under vacuum.The crude was purified by using silica gel column chromatographyusing ethyl acetate: chloroform (50:50) to yield 0.498 g (79.0percent) ofthe imatinib intermediate compound as yellowish powder. Melting point194-196 °C. ES-MS (M1) found (m/z): 308.1. 1H NMR (DMSOd6):2.49(S, 3H); 7.32-7.37 (m, 1H); 7.40-7.48 (m, 1H); 7.49e7.51(m, 1H); 7.85-7.88 (m, 1H); 8.02 (m, 1H); 8.48-8.51 (m, 1H);8.57-8.59 (m, 1H); 9.20(S, 1H); 9.29 (S, 1H). |
| 60% |
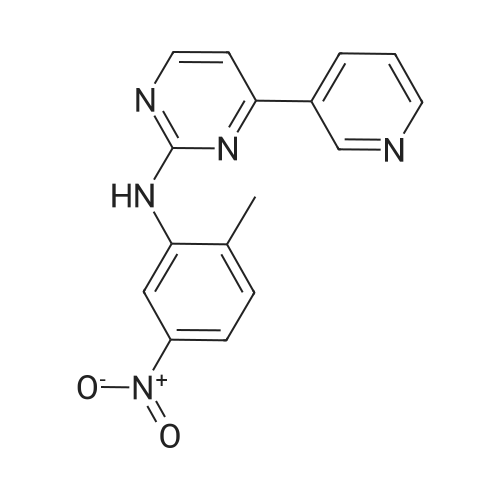
With copper(l) iodide; potassium carbonate; potassium iodide; N,N`-dimethylethylenediamine; In 1,4-dioxane; at 110℃; for 24h;Inert atmosphere; Schlenk technique; |
4-(Pyridine-3-yl) pyridine-2-amine (8.60 g, 0.05 mol), CuI (2.39 g, 0.0125 mmol), anhydrous K2CO3 (14.0 g, 0.1 mol), KI (2.08 g, 0.0125 mol) were added to a Schlenk-type three-neck flask fitted with a thermometer, magnetic stirbar, and septum. The flask was evacuated and back filled with N2 three times. Dioxane (250 mL) and 2-bromo-4-nitrotoluene (9.82 g, 0.045 mol) were added to the flask. Finally, N,N'-dimethylethylenediamine (1.10 g, 0.0125 mol) was added by syringe at room temperature with stirring. The reaction mixture was stirred at 110° C. under N2 for 24 hours and then cooled to room temperature. Ammonia (30percent, 100 mL) and saturated NaCl (400 mL), were added to the reaction mixture which was then extracted with ethyl acetate (500 mL*3). The organic phase was dried over Na2SO4 and a yellow solid was obtained after the solvent was removed under reduced pressure. The crude product was purified by column chromatography (biotage: DMC/Methanol, 1-8percent, 20 CV). The desired product was obtained as a yellow solid (8.3 g, yield: 60percent); 1H NMR (500 MHz, CDCl3) delta 2.49 (s, 3H), 7.36 (m, 1H), 7.15 (br., 1H), 7.35 (d, 1H), 7.38 (d, 1H), 7.52 (m, 1H), 7.88 (d, 1H), 8.55 (d, 1H), 8.60 (d, 1H), 8.76 (d, 1H), 9.28 (s, 1H), 9.50 (s, 1H). LC-MS (m/z) calculated, 307.3, found, 308.3 [M+H]+. |
|
With potassium tert-butylate; In dimethyl sulfoxide; at 120℃; for 12h; |
2-bromo-1-methyl-4-nitrobenzene(0.63 g, 2.9 mmol), <strong>[66521-66-2]4-(pyridin-3-yl)pyrimidin-2-amine</strong> (1) (0.60 g, 3.5 mmol), and KOtBu(0.49 g, 4.40 mmol) was added flask containing PS-CuO (57.5 mg, 20 wtpercent of CuOin PS, 0.145 mmol based on Cu) in 15 mL DMSO. The resulting mixture was heatedto 120oC and stirred for 12 h. Aftercooling, the mixture was poured into EtOAc (20.0 mL) and washed with water (2 × 10.0 mL).The organic layer was separated and dried over MgSO4, and thenevaporated under reduced pressure. The resulting mixture was reacted with stannouschloride (1.64 g, 8.70 mmol) and 1.64 mL of Con. HCl in MeOH (10 mL) at at 50 oCfor 8 h. After completion the reaction mixture was poured into crushed ice followedby basification with sodium bicarbonate. The layer was separated and dried overanhydrous MgSO4, evaporated under reduced pressure to afford the crudeproduct which was purified by using column chromatography(hexane : EtOAc = 4:1) obtainedas yellow solid 67percent (0.54 g, 1.95 mmol).1HNMR (300 MHz, CDCl3) delta 9.25 (dd, J= 1.5 Hz, 1H), 8.71 (dd, J1= 3.3 Hz, J2 = 1.5 Hz,1H), 8.48 (d, J = 5.1 Hz, 1H), 8.35-8.31(m, 1H), 7.59 (d, J = 2.1 Hz,? 1H), 7.43 ? 7.38 (m, 1H), 7.12 (d, J = 5.4Hz,1H) 7.03(bs, 1H), 6.98 (d, J = 8.1 Hz, 1H), 6.41 (dd, , J1 = 5.7 Hz, J2= 2.4 Hz, 1H), 3.59(bs, 2H), 2.24(s, 3H). 13C NMR (75 MHz, CDCl3): delta 162.5, 160.7, 159.0, 151.4, 148.5, 145.1,137.9, 134.4, 132.7, 131.0, 123.5, 118.1, 110.6, 108.4, 108.0 HRMS(ESI) calcd. for C16H16N5 [M+H]+ 278.1406, found 278.1400. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping