|
|
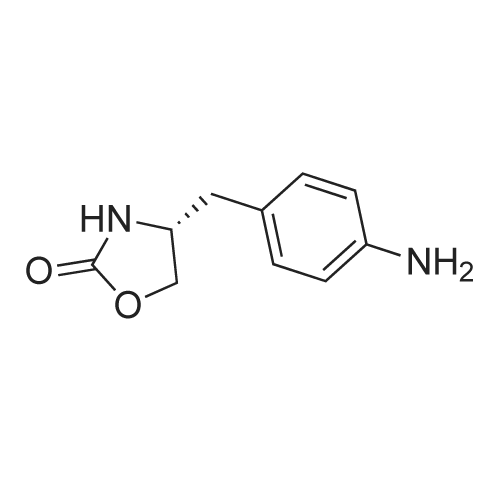
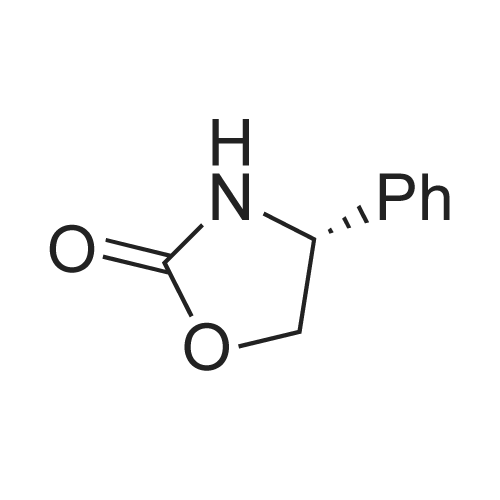
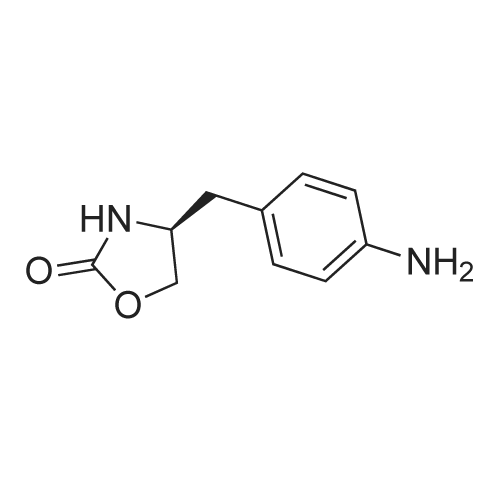
2.4 litres of concentrated HCl are added to 7.0 litres of water in a duplicator. In such diluted HCl 1.75 kg of (<S)-4-(4-aminobenzyl)-l,3-oxazolidine-2-one of formula (I) is stirred up and the mixture is cooled down to 0C under stirring. During the cooling an aqueous solution ofNaNO2 (0.64 kg NaNO2 / 1.4 litres of water) is prepared for diazotation. At the temperature of ca. O0C a solution OfNaNO2 / H2O is slowly added to the solution of the starting substance (I) on intensive stirring and cooling. After adding of all the nitrite the mixture is further stirred at ca. O0C for ca. 30 min. Example 2 - Reduction of the diazonium saltWeighed 0.28 kg of NaOH is dissolved in 7.0 litres of water and of 2.66 kg of sodium disulphite are added under stirring. After dissolution the mixture is cooled to ca. 20C. The diazonium salt solution is slowly added to the cooled disulphite solution under intensive stirring at the temperature of 2 - 5C. The mixture is then stirred at ca. 5C for additional 30 minutes. Then, it is heated up to ca. 25C and stirred at this temperature for 45 minutes.Further it is heated under a reflux condenser to ca. 6O0C while being stirred. After heating 900 ml of concentrated HCl is added to the reaction mixture and the mixture is stirred under the reflux condenser for another 5 hours. Then the mixture is diluted with 14 litres of water. <n="7"/>Example 3 - Fischer indole reactionThe diluted reaction mixture is heated up to ca. 90C. 1.75 kg of 4,4-diethoxy-N,N- dimethylbutylamine of formula II is weighed. The weighed acetal (II) is added to the reaction mixture, which is then brought to moderate reflux (at ca. 980C) and being stirred under the reflux condenser it is left to react for about 2.5 hours. After 2.5 hours from the start of the reflux the heating of the mixture is switched off and the reaction mixture is cooled to the laboratory temperature.Example 4 - Isolation of the raw toluene solvate of ZOLMITRIPTANAt the laboratory temperature and being stirred the cooled mixture is neutralized with a ca. 20% aqueous solution of NaOH. About 4 litres of toluene is added and the mixture is stirred to make an emulsion. Then, an aqueous solution of NaOH is slowly added under stirring until pH of ca. 9.5 is achieved. After approx. 30 minutes of stirring the solid product is filtered off and washed with water. It is dried at temperatures of about 300C to the constant weight (about 10 hours). |
|
|
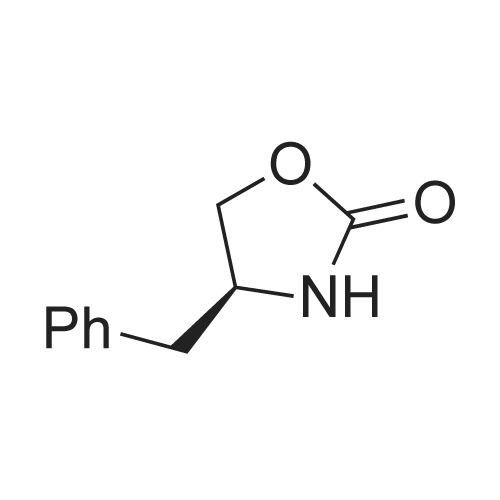
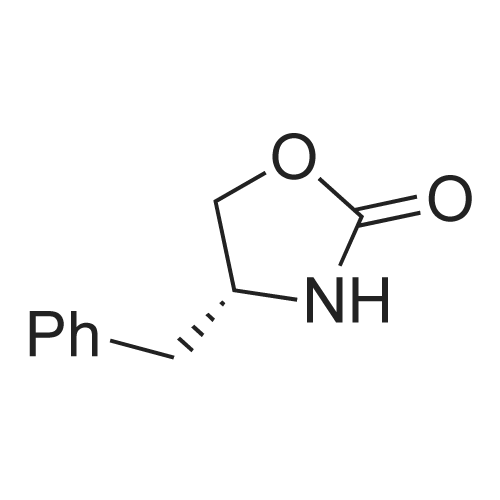
Sodium nitrite (16 gm) in water (120 ml) was added slowly for a period of 30 minutes at 0 deg C to a solution of (S)-4-(4-Aminobenzyl)-2-oxazolidinone (40 gm), concentrated hydrochloric acid (46 ml) and water (480 ml) in round bottomed flask, cooled to 0 deg C and stirred for 1 hour. The above diazotized solution was added for a period of 30 minutes at 0 deg C to sodium sulfite (78.3 gm) in water (200 ml) in another round bottomed flask, cooled to 0 deg C, slowly allowed to room temperature, heated to 55 deg C and stirred for 15 minutes at 60 deg C. Added concentrated hydrochloric acid (80 ml) to the reaction mass, stirred for 16 hours at 60 deg C, nitrogen gas was applied and heated to 90 deg C. Water (80 ml) was added to the reaction mass for 15 minutes at 90 deg C, added 4-(dimethylamino)-butyraldehyde diethylacetal for a period of 40 minutes, heated to reflux, stirred for 3 hours at reflux, cooled to 25 - 30 deg C and the pH was adjusted to 7 by adding sodium hydroxide solution(30%, 230 ml). Extracted with ethyl acetate (7 X 200 ml), adjusted the pH of the aqueous layer to 10 by adding sodium hydroxide solution (30%, 100 ml), heated to 50 deg C and again extracted with ethyl acetate (8 X 200 ml) at 50 deg C. Both the organic layers were combined, dried with sodium sulfate, given carbon treatment and the solvent was distilled off completely under vacuum at 50 - 55 deg C, ethyl acetate (80 ml) was added to the reaction mass at 25 deg C, stirred for 1 hour and cooled to 10 deg C. Stirred for 30 minutes at 10 deg C, filtered the material and washed with chilled ethylacetate(20 ml) under nitrogen atmosphere and dried at 45 -50 deg C to yield 40 gm of (4S)-4-[[3-[2-(Dimethylamino)ethyl]~ 1 H-indol-5-yl]methyl]-2-oxazolidinone.(4S)-4-[[3-[2-(Dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone (40 gm, Zolmitriptan) was dissolved in isopropanol (200 ml) at 25 deg C, heated to reflux, stirred for 40 minutes at reflux and slowly allowed to cool to 0 deg C. Stirred the reaction mass for 1 hour at 0 deg C, filtered the compound, washed with chilled isopropanol(40 ml) and dried at 40 - 45 deg C under vacuum to yield (4S)-4-[[3-[2-(Dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone isopropanol solvate (32 gm, Zolmitriptan isopropanol solvate; HPLC purity. 99.32%). Zolmitriptan isopropanol solvate obtained above (32 gm) was dissolved in isopropyl acetate (2250 ml) at 25 deg C. Then the contents were heated to reflux and maintained for 30 minutes to form clear solution. The solution was cooled to 25 deg C during a period of 1 hour. The separated solid was filtered, washed with isopropyl acetate (160 ml) to obtain 32 gm of zolmitriptan isopropyl acetate solvate (HPLC purity: 99.8%). |
|
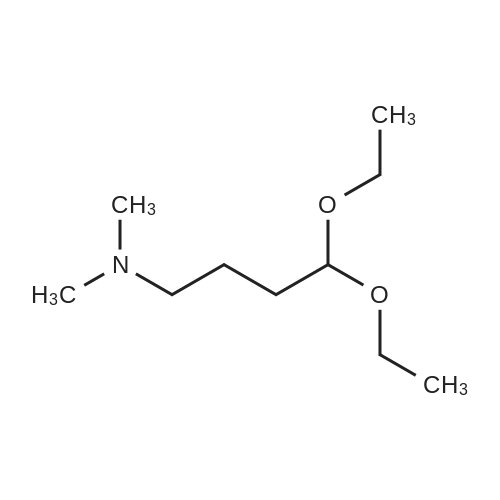
|
Example - 1;The preparation of (SV4-[(3-[2-(DimethgammalaminokthyIl-lH-indol-5-gammal1methvn-2- oxazolidinone (I):Charge to the flask 300 ml of cone, hydrochloric acid, 3L of demineralised water and 250 gms of (S)-4-(4-arninobenzyl)-2-oxazolidinone. Cool the reactor contents to between 0-5 DEG C and add aqueous sodium nitrite solution (99 gms in 900ml water), maintaining the temperature below 5 DEG C. After stirring for about 30 minutes add the diazonium salt solution to a chilled aqueous solution of sodium sulphite (492 gms in 800 ml water) maintaining the temperature below 10 DEG C. After stirring for 15 minutes slowly heat the resulting mixture to about 55-60 DEG C, and then slowly add 50 ml of cone. hydrochloric acid. The solution is maintained at about 60 DEG C for about 18 hours. Dilute the reaction mixture with 5L of water and heat to about 90 DEG C. Under a nitrogen atmosphere slowly add 246 gms of 4,4-diethoxy-N, N-dimethylbutylamine and heat at reflux for about 3 hours. Cool, and adjust the mixture to about pH7 using sodium hydroxide solution. Extract with chloroform and then adjust the aqueous layer to about pHIO, again using sodium hydroxide solution. Extract the product using chloroform. Treat the combined chloroform extracts (containing the product) with decolorizing charcoal, and filter through filter aid. Distil off most of the solvent and chill the suspension to about 0 DEG C. Centrifuge the crude product, wash with chloroform and vacuum dry at 50 DEG C. (150 gms, pale yellow powder, 99.4% HPLC purity) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping