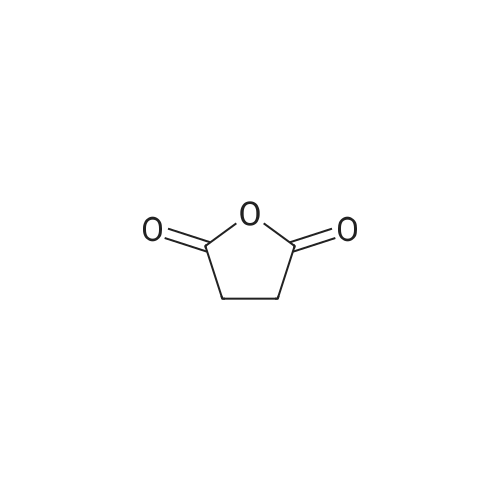
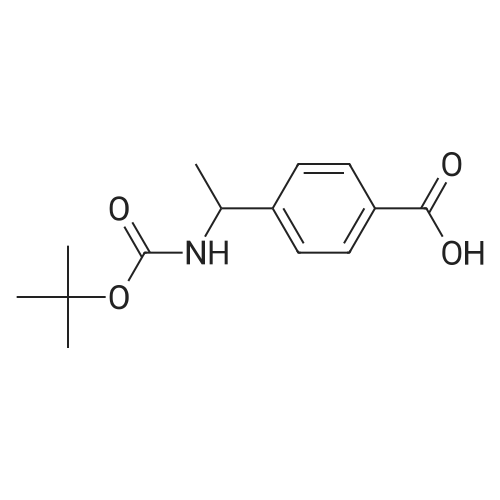
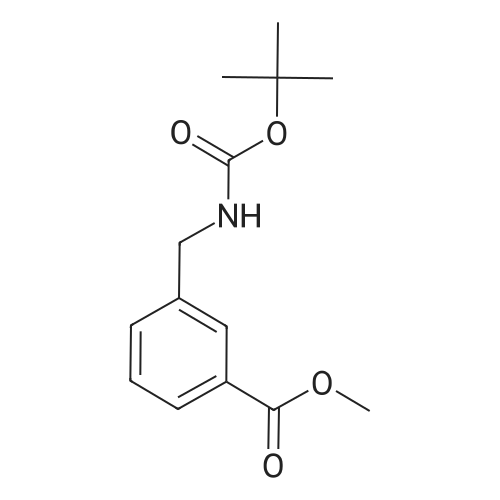
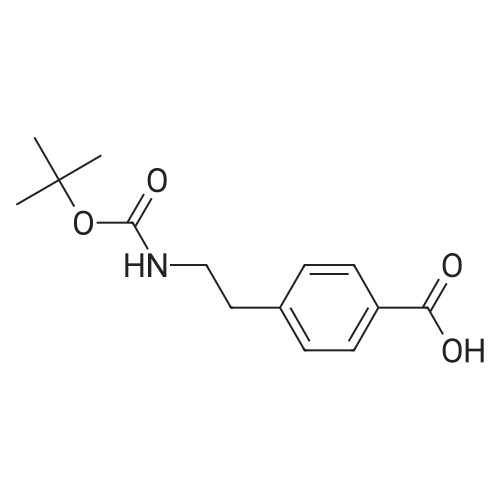
| 70% |
With ethanol; lithium hydroxide; at 35.0℃; for 1.0h; |
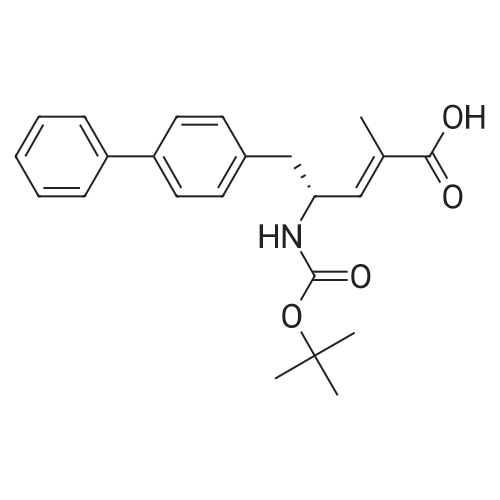
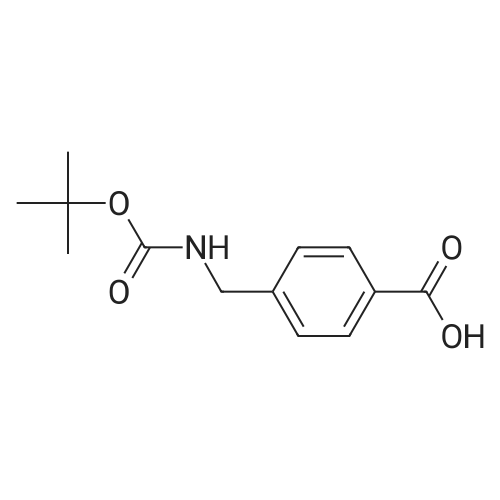
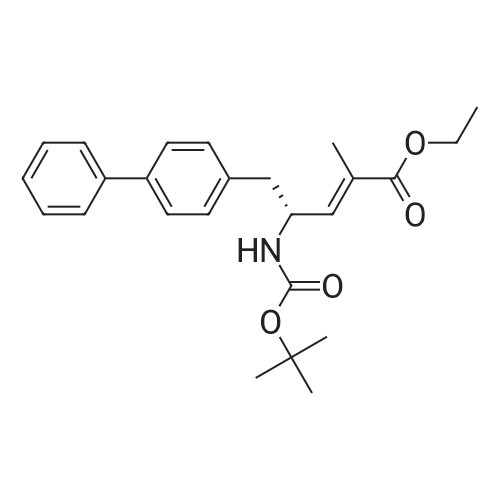
6) To 94.6g (4R) -5- [1,1'-biphenyl] -4-yl-4-[[tert-butoxycarbonyl] amino] -2-methyl-2-pentenoic acid ethyl esterAdd 473mL ethanolAnd 13.7g of lithium hydroxide, stirred at 35 for 1h,After the reaction, the crystals were concentrated to obtain 61.4g(R, E) -5-([1,1'-biphenyl] -4-yl) -4-((tert-butoxycarbonyl) amino) -2-methyl-2-pentenoic acid;(70% yield); |
|
With lithium hydroxide; ethanol; |
Example 1: (E)-(R)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid; [Show Image] (E)-(R)-5-Biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid ethyl ester (CASNo. 149709-59-1) is hydrolysed using lithium hydroxide in ethanol to yield (E)-(R)-5-biphenyl-4-yl-4-tert-butoxycarbonylamino-2-methylpent-2-enoic acid. White solid. deltaH (400 MHz; DMSO) 1.31 (9H, s, (CH3)3), 1.59 (3H, s, 1-CH3), 2.68 (1H, dd, J 6.8, 13.2, 5-HA), 2.86 (1H, m, 5-HB), 4.44 (1H, m, 4-H), 6.51 (1H, d, J 9.2, 3-H), 7.16 (1H, d, J 8.0, NH), 7.26 (2H, d, J 8.0, Ar-ortho-H(Ph)), 7.31 (1H, t, J 7.6, Ar-(Ph)-para-H), 7.40 (2H, t, J 8.0, Ar-(Ph)-meta-H), 7.54 (2H, d, J 8.0, Ar-meta-H(Ph), 7.60 (2H, d, J 7.6, Ar-(Ph)-ortho-H), 12.26 (1H, s, CO2H); m/z (+ESI) 404 ([MNa]+, 17%), 382 ([MH]+, 2), 326 (10), 264 (100), 167 (13). |
|
With lithium hydroxide; at 80.0℃; for 1.0h; |
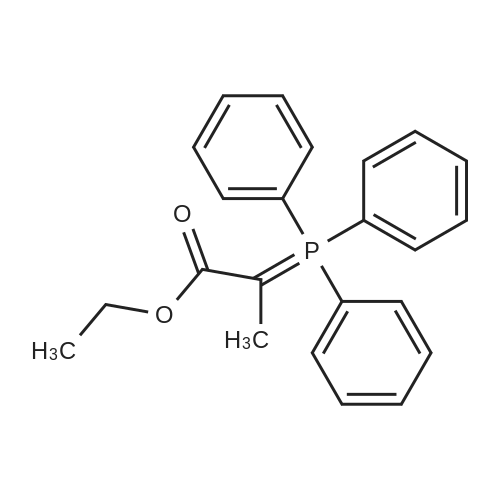
0.1833 mol of compound I,1 L of isopropyl acetate,1.00 mol of NaBr,Was added to a 2 L flask,Stirring at 20 C for 30 min,Then the temperature is controlled to 20 C,Add TEMPO;Step 2 Preparation of NaClO-NaHCO3 aqueous solution:1.25 mol of NaHCO3 was dissolved in 360 ml of water,An aqueous NaCl solution containing 0.220 mol of available chlorine was added dropwise to the solution at a temperature of 10-15 C;Step 3 An aqueous solution of NaClO-NaHCO3 prepared in Step 2 was added dropwise to the isopropyl acetate solution of Compound I-NaBr,10-15 temperature drop in 80min,And then adding sodium thiosulfate solution to terminate the reaction,Layered organic phase,Washed with aqueous NaCl solution,To obtain the isopropyl acetate solution of compound II;Step 4 To a solution of compound II in isopropyl acetate,Add phosphorusYe Lide,30 reaction 1h,Add a water to the lemonAcid terminates the reaction,And insulation 0.5h.Dispensing,Washed with organic phase,The compound III was distilled under reduced pressure;Step 5 To the resulting compound III was added 1.3 mol of lithium hydroxide,80 insulation reflux 1h,Cooling crystallization,Filter,Dried to obtain 56.1 g of compound IV dry product,The molar yield was 80.25%Purity 99.30%.TEMPOtempDefinitions of temponounthe speed at which a passage of music is or should be played.Listening to music with a slow tempo helps calm the mind.synonyms: speed, cadence, rhythm, beat, time, pulse, measure, meterthe rate or speed of motion or activity; pace.the tempo of life dictated by a heavy workloadsynonyms: pace, rate, speed, velocityTranslations of temponounspeed, rate, velocity, pace, tempo, quicknessbeat, tempo, time, racket, racquet, tempitempi, tempotempoGoogle Translate for Business:Translator ToolkitWebsite TranslatorGlobal Market Finder |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping