| 76% |
With ammonia; In methanol; at 25 - 40℃; for 40h; |
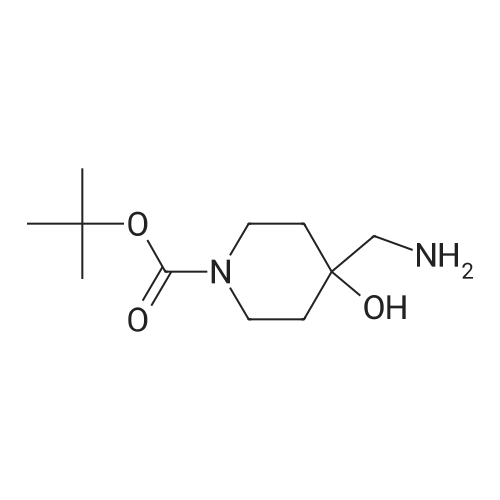
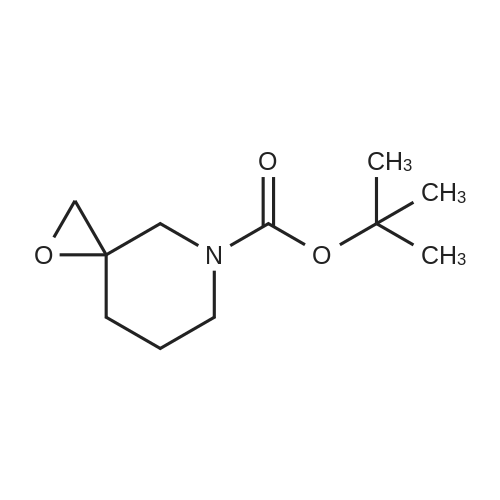
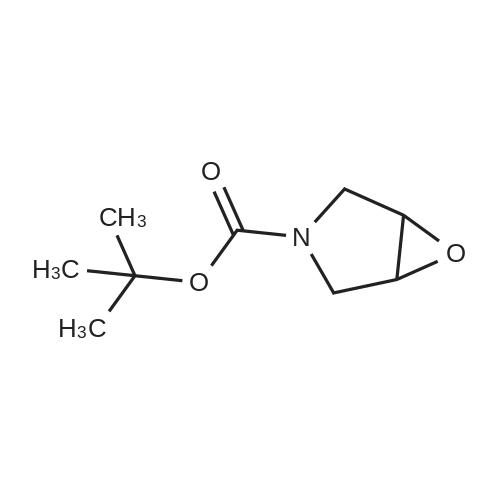
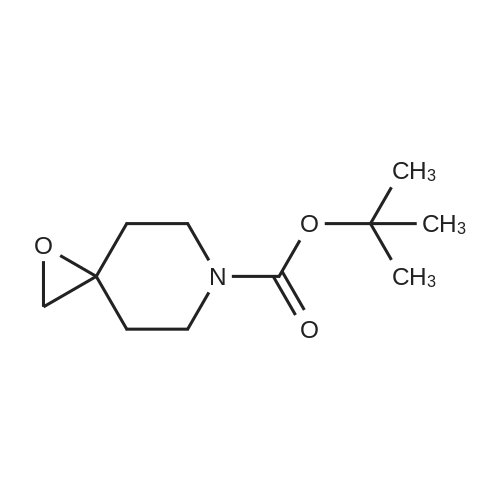
tert-Butyl l-Oxa-6-aza-spiro[2.5]octane-6-carboxylate (0.5 grams, 2.34 mmole) was added to methanolic ammonia solution (20 mL, 14.83 % w/v) at room temperature. Then reaction mass was stirred for 40 hours at room temperature in a closed vessel. The progress of the reaction was monitored by TLC. After completion of the reaction (TLC), the reaction mass was concentrated on rotavacuum to obtain the title compound. Yield: 0.41 gram (76%). 'H-NMR(8 ppm): 1.35 - 1.69 (16H, m), 2.61 (2H, s), 3.10 - 3.20 (2H, m), 3.81 -3.90(2H, m); Mass (m/z): 231.3 (M+H)+. |
| 76% |
With ammonia; In methanol; at 20℃; for 40h; |
tert-Butyl l-oxa-6-azaspiro[2.5]octane-6-carboxylate (0.5 grams, 2.34 mmole, obtained in the step (i) of preparation 5) was added to methanolic ammonia solution (20 mL, 14.83 % w/v) at RT. Then reaction mass was stirred for 40 hours at RT in a closed vessel. The reaction mass was concentrated under vacuum to obtain the title compound. Weight: 0.41 gram (Yield: 76 %). (0385) - NMR (delta ppm): 1.35 - 1.69 (16H, m), 2.61 - 2.69 (2H, m), 3.10 - 3.20 (2H, m), 3.81 - 3.90 (2H, m); (0386) Mass (m/z): 231.3 (M+H)+ . |
| 69% |
With ammonia; In methanol; at 20℃; |
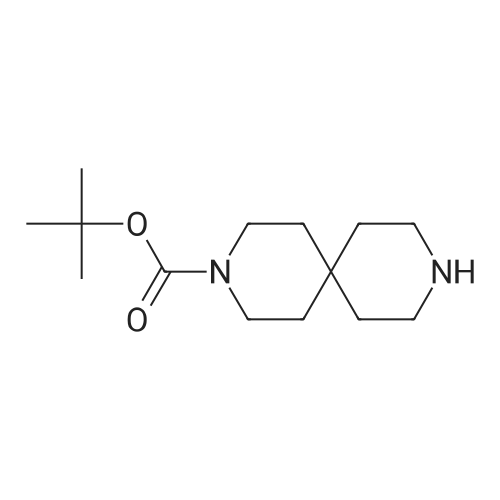
Intermediate 2M: tert-butyl 4-(amiriomethyl)-4-hydroxypiperidine-1 -carboxylate A mixture of intermediate 1A (10.0 g, 46.9 mmol) and ammonia solution (201 mL, 7 M solution in methanol, 1.4 mol) was stirred at r.t. overnight. The solvent was removedunder vacuum and the residue was purified by flash chromatography, silica gel, gradient dichioromethane to methanol:dichloromethane (1:4) to give the title compound (7.4 g, 69% yield) as a white solid. HPLC retention time: 2.15 mm; MS:131 (M+H-100). |
| 69% |
With ammonia; In methanol; at 20℃; |
A solution of intermediate 1A (10.0 g, 46.9 mmol) in ammonia (201 ml_, 7 M solution in methanol, 1.4 mol) was stirred at r.t. overnight. The solvent was removed under vacuum and the residue was purified by flash chromatography, silica gel, gradient dichloromethane to methanokdichloromethane (1 :4) to give the title compound (7.4 g, 69% yield) as a white solid. HPLC retention time: 2.15 min; MS: 131 (M+H-100). |
| 69% |
With ammonia; In methanol; at 20℃; |
Intermediate 2A: fert-butyl 4-(aminomethyl)-4-hydroxypiperidine-1-carboxylate A mixture of intermediate 1A (10.0 g, 46.9 mmol) and ammonia solution (201 mL, 7 M solution in methanol, 1.4 mol) was stirred at r.t. overnight. The solvent was removed under vacuum and the residue was purified by flash chromatography, silica gel, gradient dichloromethane to methanokdichloromethane (1 :4) to give the title compound (7.4 g, 69% yield) as a white solid. HPLC retention time: 2.15 min; MS: 131 (M+H-100). |
| 60.5% |
With ammonia; In ethanol; water;Inert atmosphere; |
A stirred solution of 1-oxa-6-aza-spiro[2.5]octane-6-carboxylic acid tert-butyl ester (9.0 g, 42.2 mmol) in ethanol (60 ml) was added with ammonia (100 ml). The resulting mixture was stirred under a nitrogen atmosphere overnight. The mixture was concentrated under reduced pressure. The residue was added with water (50 ml) and extracted with ethyl acetate (150 ml*3). The combined organic extracts were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with dichloromethane:methanol (20:1) as eluents to give 4-aminomethyl-4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester (5.87 g, 60.5%) as a white solid. MS m/z (ESI): 231 [M+1] |
| 60.5% |
With ammonia; In ethanol; |
A stirred solution of l-oxa-6-aza-spiro [2.5] octane-6-carboxylic acid tert-butyl ester (9.0 g, 42.2 mmol) in ethanol(60 ml) was added with ammonia (100 ml). The resulting mixture was stirred under a nitrogen atmosphere overnight. The mixture was concentrated under reduced pressure. The residue was added with water (50 ml) and extracted with ethyl acetate (150 ml><3). The combined organic extracts were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with dichloromethane: methanol (20:1) as eluents to give 4-aminomethyl-4-hydroxy-piperidine-l-carboxylic acid tert-butyl ester (5.87 g, 60.5%) as a white solid. MS m/z (ESI): 231[M+1] , |
|
With ammonia; In methanol; water; at 0 - 20℃; for 7h; |
4-Aminomethyl-4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester is prepared from 1-oxa-6-aza-spiro[2.5]octane-6-carboxylic acid tert-butyl ester (Bourrain et al Bioorg. Med. Chem. Lett. 9(23):3369-3374 (1999)). Concentrated aqueous NH4OH (6 mL) is added to a solution of 1-oxa-6-aza-spiro[2.5]octane-6-carboxylic acid tert-butyl ester (0.50 g, 2.3 mmol) in MeOH (4 mL) at 0 C. The reaction mixture is removed from the cooling and allowed to warm to room temperature. After 7 hours, the reaction mixture is concentrated under reduced pressure to afford desired product as a white solid. |
|
With ammonium hydroxide; In methanol; at 0 - 20℃; for 16h; |
aqueous ammonia (6mL)was added to a solution of tert-butyl 1-oxa-6-azaspiro[2.5]octan-6-carboxylate (500mg, 2mmol) in methanol (4mL) at0C. The reaction solution warmed to room temperature and was stirred for 16 hours, then concentrated under reduced pressure to give a colorless oil (530mg, 2.3mmol) with a yield of 100%, which was used directly in the next step withoutpurification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping