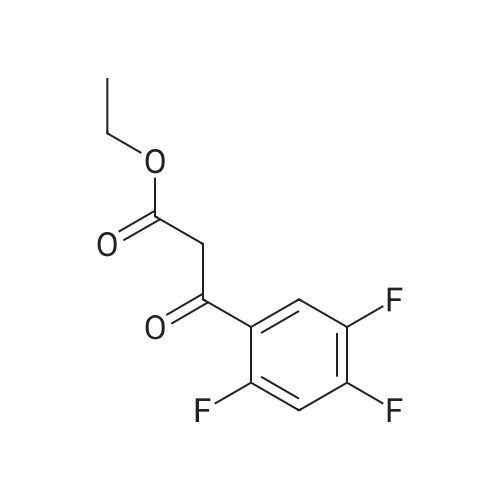
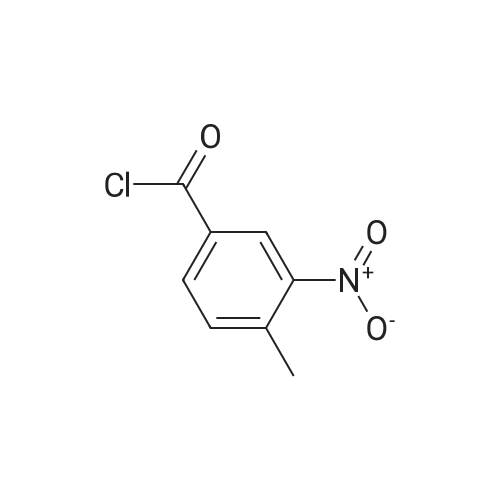
| 25.2 mg |
With lithium hexamethyldisilazane; In tetrahydrofuran; for 0.916667h;Cooling with acetone-dry ice; |
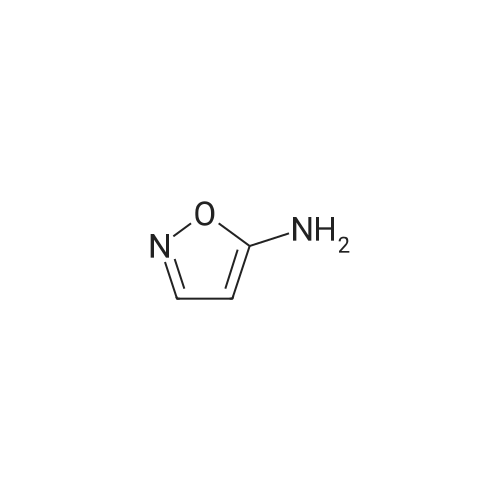
A round bottom flask was charged with perfluorophenyl 4-(2-cyano-4-(trifluoromethyl)phenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonate (44.1 mg, 0.080 mmol), <strong>[14678-05-8]isoxazol-5-amine</strong> (10.1 mg, 0.12 mmol, Matrix Scientific, Columbia, SC) and THF (1 mL) to give a a clear, colorless solution. The flask was cooled in a dry ice-acetone bath for 5 min, then lithium bis(trimethylsilyl)amide (1M in THF) (168 mu, 0.168 mmol) was added dropwise over 30 s to give a light-yellow solution. After 10 min, the flask was lowered into an ice bath for 45 min. Glacial acetic acid (1 drop) was added, and the mixture was concentrated under a vacuum. The product was purified by chromatography on silica gel (12g column with 0 to 10% MeOH/DCM) to give 4-(2-cyano-4-(trifluoromethyl)phenyl)-N-(isoxazol-5-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonamide (25.2 mg, 0.056 mmol) as a light-yellow solid. 1H NMR (400MHz, DMSO-d6) delta ppm 12.18 (br. s., 1 H), 8.42 (d, J= 2.1 Hz, 1 H), 8.34 (d, J= 2.0 Hz, 1 H), 8.11 (dd, J= 2.0, 8.9 Hz, 1 H), 7.76 (d, J= 8.6 Hz, 1 H), 7.30 (d, J= 2.2 Hz, 1 H), 7.23 (dd, J= 2.2, 8.7 Hz, 1 H), 6.80 (d, J= 8.6 Hz, 1 H), 5.77 (d, J= 1.9 Hz, 1 H), 4.40 - 4.35 (m, 2 H), 3.89 - 3.86 (m, 2 H). m/z (ESI) 451.4 (M+H)+. |
| 25 mg |
With lithium hexamethyldisilazane; In tetrahydrofuran; for 0.916667h;Cooling with acetone-dry ice; |
A round bottom flask was charged with perfluorophenyl 4-(2-cyano-4-(trifluoromethyl)phenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonate (44 mg, 0.080 mmol), <strong>[14678-05-8]isoxazol-5-amine</strong> (10 mg, 0.12 mmol, Matrix Scientific, Columbia, SC) and THF (1 mL) to give a a clear, colorless solution. The flask was cooled in a dry ice-acetone bath for 5 min, then lithium bis(trimethylsilyl)amide (1M in THF) (170 mul, 0.170 mmol) was added dropwise over 30 s to give a light-yellow solution. After 10 min, the flask was lowered into an ice bath for 45 min. Glacial acetic acid (1 drop) was added, and the mixture was concentrated under a vacuum. The product was purified by chromatography on silica gel (12g column with 0 to 10% MeOH/DCM) to give 4-(2-cyano-4-(trifluoromethyl)phenyl)-N-(isoxazol-5-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-sulfonamide (25 mg, 0.056 mmol) as a light-yellow solid. 1H NMR (400MHz, DMSO-d6) delta ppm 12.18 (br. s., 1 H), 8.42 (d, J = 2.1 Hz, 1 H), 8.34 (d, J = 2.0 Hz, 1 H), 8.11 (dd, J = 2.0, 8.9 Hz, 1 H), 7.76 (d, J = 8.6 Hz, 1 H), 7.30 (d, J = 2.2 Hz, 1 H), 7.23 (dd, J = 2.2, 8.7 Hz, 1 H), 6.80 (d, J = 8.6 Hz, 1 H), 5.77 (d, J = 1.9 Hz, 1 H), 4.40 - 4.35 (m, 2 H), 3.89 - 3.86 (m, 2 H). m/z (ESI) 451.4 (M+H)+. HRMS m/z Calculated for C19H14F3N4O4S [M+1]+ = 451.0688. Found [M+1]+ = 451.0685. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping