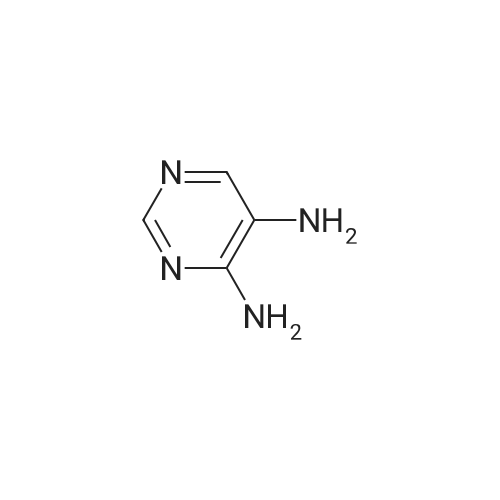
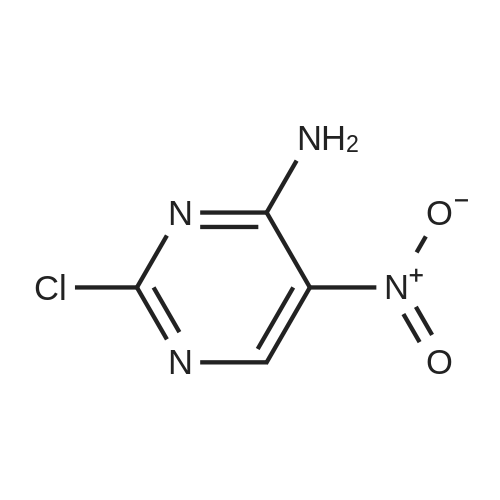
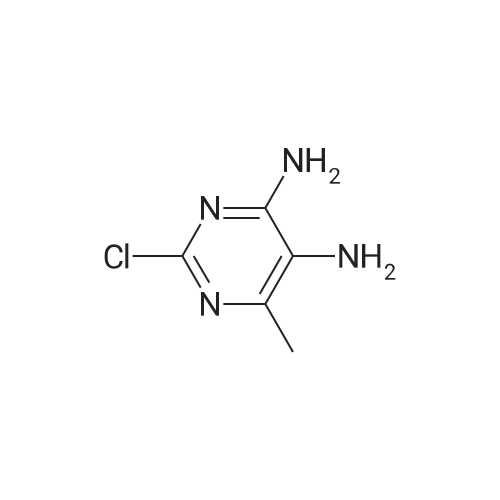
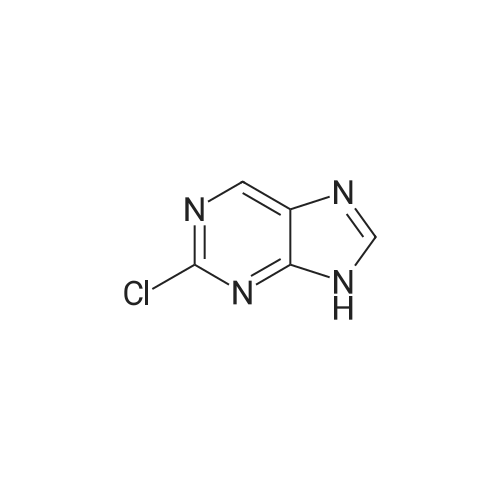
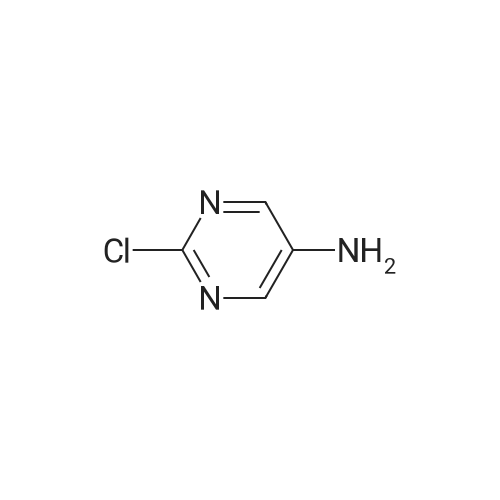
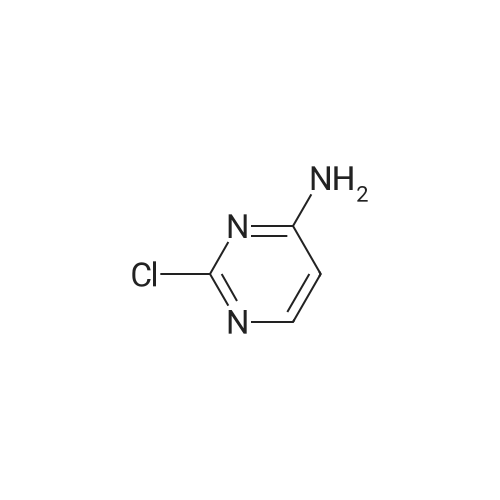
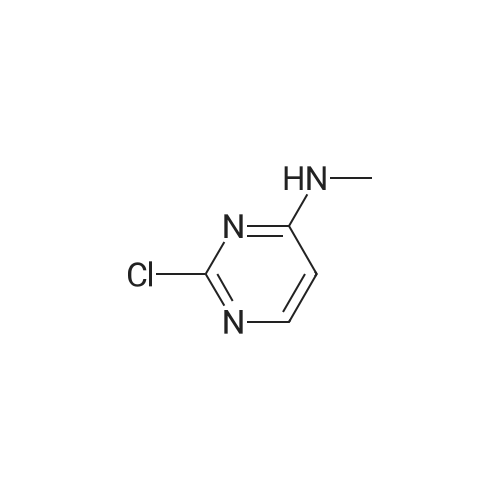
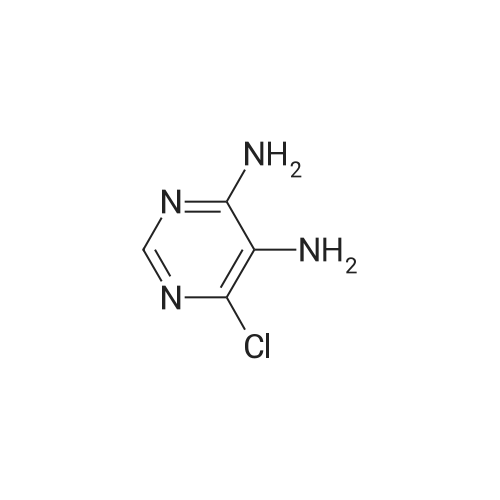
| 54.5% |
With water; iron; ammonium chloride; In ethanol; at 100℃; for 4h; |
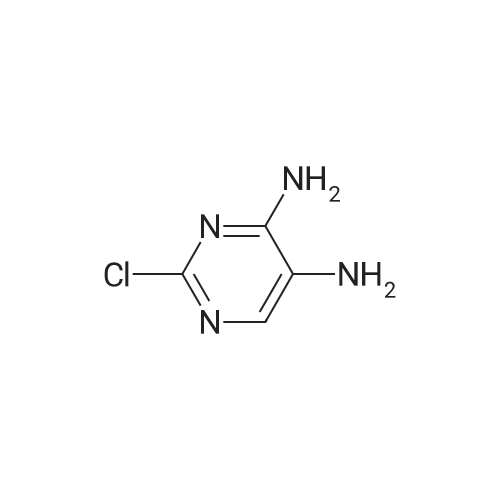
To a solution of compound 21-2 (20 g, 114.6 mmol) in EtOH/H2O (4/1, 400 mL) was added iron powder (64 g, 1146 mmol) and NH4Cl (aq., 62 g, 1146 mmol), the reaction solution was stirred at 100 C. for 4 h, followed by filtration. The filter cake was washed with MeOH (10 mL×3) and the filtrate was concentrated to give compound 21-3 (9 g, Yield 54.5%) as yellow solid. |
| 49% |
With tin(II) chloride dihdyrate; In ethanol; at 80℃; for 2h;Inert atmosphere; |
Step-1: 2-chloropyrimidine-4,5-diamine [0176] A mixture of <strong>[1920-66-7]2-chloro-5-nitropyrimidin-4-amine</strong> (1.0 g, 5.7 mmol) and SnCl2·2H2O (5.2 g, 22.9 mmol) in EtOH (55 mL) under N2 was heated to 80 C and stirred for two hours. The mixture was then concentrated under reduced pressure. EtOAc and Celite were added to the residue and the mixture was basified with saturated Na2CO3 (aq.) to pH 9- 10. The mixture was filtered through a pad of Celite and washed with EtOAc. The organic layer was separated and washed with brine, dried (Na2SO4) and concentrated in vacuum. The residue was purified on ISCO (20 g silica gel column, EtOAc/hexanes 0~100%) to give the title compound (0.41 g, 49%). |
| 33% |
With iron; ammonium chloride; In tetrahydrofuran; ethanol; water;Reflux; |
General procedure: Commercial 1a (50mmol) was dissolved in dichloromethane (80mL), mixture was cooled to 0C, solution of NH3 in MeOH (4mol/L, 12.5mL) was added by dripping slowly, after 30min, the mixture maintained at 0C for 1h. The reaction mixture was filtered, and the insoluble material was washed with ethyl acetate (20mL) and water (30mL) to get 2a (8.66g), yield 90%. Compound 2a (50mmol) was dissolved in THF (40mL), then absolute ethyl alcohol (20mL), water (20mL), Fe (4eq) and ammonium chloride (2eq) were added. The mixture was refluxed until raw material disappeared. The reaction mixture was filtered and filter liquor was evaporated by reducing pressure to get 3a (2.4g), yield 33%. Compound 3a (17mmol) was dissolved in DMF (30mL), triethylamine (3eq) was added, and acetyl isothiocyanate (1eq) was added by dripping slowly, the mixture was stirred at room temperature for 30min, then 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 1.5eq) was added and stirred overnight at room temperature. Pulled the mixture into ice water, using concentrated HCl regulates pH to 1, the mixture was filtered, the residue was dried and washed by water to give 4a (2.0g), yield 60%. Compound 4a (10mmol), 5a/5b/5c/5d/5e (1.2eq), 120mL 1,4-dioxane, 10mL water, Pd(dppf)Cl2 (0.1eq), and NaHCO3 (1eq) were added to a round-bottomed flask, mixture was stirred for 12h at 90C. Solvent was removed by reduced pressure distillation, residue was purified by preparative HPLC (MeCN+0.05% TFA, H2O 0.1%+TFA), Ta-e were finally obtained from above steps, yield 0.58-5.5%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping