|
With n-butyllithium; In tetrahydrofuran; hexane; water; |
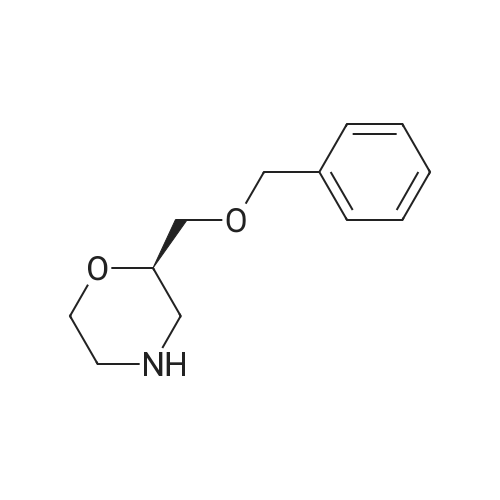
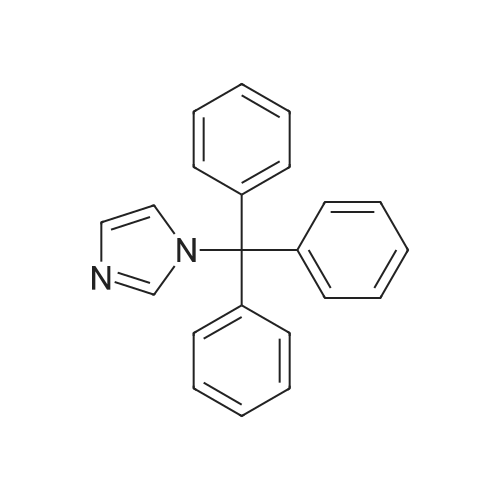
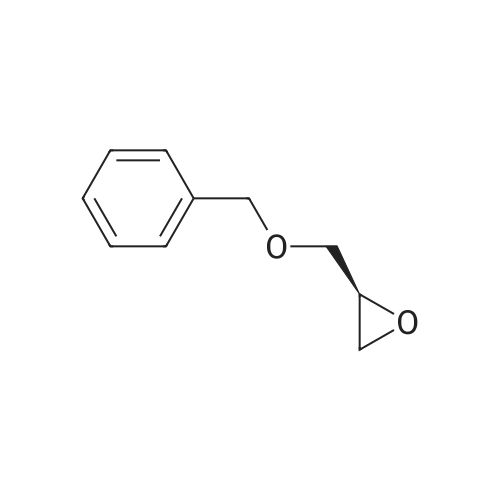
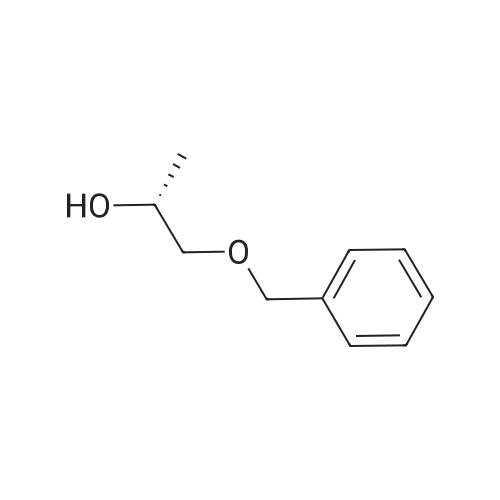
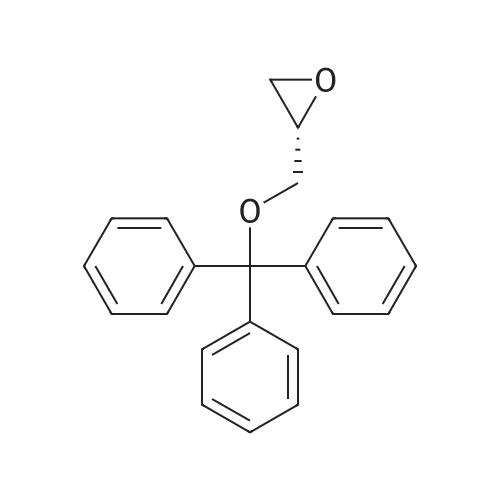
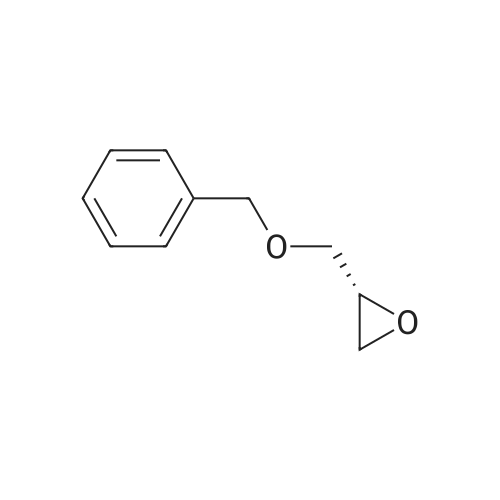
(i) (2R)-1-(benzyloxy)-3-(1-trityl-1H-imidazol-2-yl)-2-propanol In an argon atmosphere, n-butyllithium (1.6 M solution in hexane, 6.9 ml) was added drop by drop to a solution of 1-tritylimidazole (3.10 g) in THF (80 ml) under ice cooling. After stirring at the same temperature for 30 minutes, (R)-2-[(benzyloxy)methyl]oxirane (1.52 ml) was added. After stirring under ice cooling for 1.5 hours and at room temperature for 1 hour, water was added and the reaction mixture was extracted with ethyl acetate. The extract was washed with water and saline and dried over magnesium sulfate, after which it was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent: ethyl acetate-hexane 1:1) to yield the titled compound (1.402 g) as a pale-yellow oily substance. 1H-NMR (CDCl3) delta: 2.06 (2H, dd, J=2.8 Hz, 18.0 Hz) 3.08 (1H, dd, J=5.4 Hz, 9.8 Hz), 3.21 (1H, dd, J=5.4 Hz, 9.8 Hz), 3.55-3.7 (1H, m), 4.36 (2H, s), 6.73 (1H, d, J=1.4 Hz), 6.93 (1H, d, J=1.4 Hz), 7.0-7.4 (20H, m). |
|
With n-butyllithium; In tetrahydrofuran; hexane; water; |
(i) (2R)-1-(benzyloxy)-3-(1-trityl-1H-imidazol-2-yl)-2-propanol In an argon atmosphere, n-butyllithium (1.6 M solution in hexane, 6.9 ml) was added drop by drop to a solution of 1-tritylimidazole (3.10 g) in THF (80 mL) under ice cooling. After stirring at the same temperature for 30 minutes, (R)-2-[(benzyloxy)methyl]oxirane (1.52 mL) was added. After stirring under ice cooling for 1.5 hours and at room temperature for 1 hour, water was added and the reaction mixture was extracted with ethyl acetate. The extract was washed with water and saline and dried over magnesium sulfate, after which it was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent: ethyl acetate-hexane 1:1) to yield the titled compound (1.402 g) as a pale-yellow oily substance. 1H-NMR (CDCl3) delta: 2.06 (2H, dd, J=2.8 Hz, 18.0 Hz), 3.08 (1H, dd, J=5.4 Hz, 9.8 Hz), 3.21 (1H, dd, J=5.4 Hz, 9.8 Hz), 3.55-3.7 (1H, m), 4.36 (2H, s), 6.73 (1H, d, J=1.4 Hz), 6.93 (1H, d, J=1.4 Hz), 7.0-7.4 (20H, m). |
|
|
n-butyllithium (1.6M hexane solution, 6.9ml) was added dropwise to a solution of 1-tritylimidazole (3.10g) in THF (80mL) under ice-cooling in the argon atmosphere. After stirred at the same temperature for 30 minutes, (R)-2-[(benzyloxy)methyl]oxirane (1.52mL) was added. After stirred for 1.5 hours under ice-cooling and at room temperature for 1 hour, water was added to the reaction solution, followed by ethyl acetate. The extract was washed with water and a brine, dried with magnesium sulfate, and concentrated under reduced pressure. The residue was purified by subjecting to the silica column gel chromatography (eluent; ethyl acetate:hexane= 1:1) to obtain the title compound (1.402g) as a pale yellow oil. 1H-NMR (CDCl3) delta: 2.06 (2H, dd, J = 2.8Hz, 18.0Hz), 3.08 (1H, dd, J = 5.4Hz, 9.8Hz), 3.21 (1H, dd, J = 5.4Hz, 9.8Hz), 3.55-3.7 (1H, m), 4.36 (2H, s), 6.73 (1H, d, J = 1.4Hz), 6.93 (1H, d, J = 1.4Hz), 7.0-7.4 (20H, m). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping