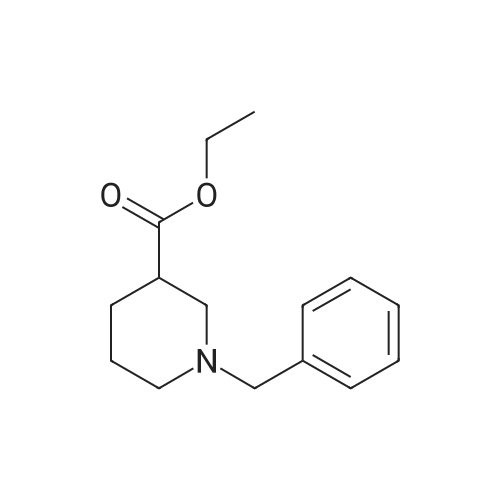
| 61.4% |
|
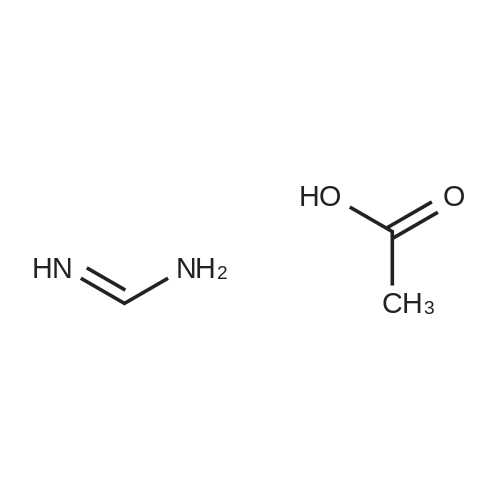
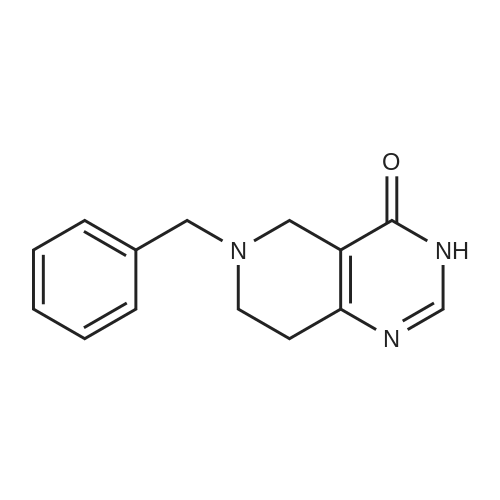
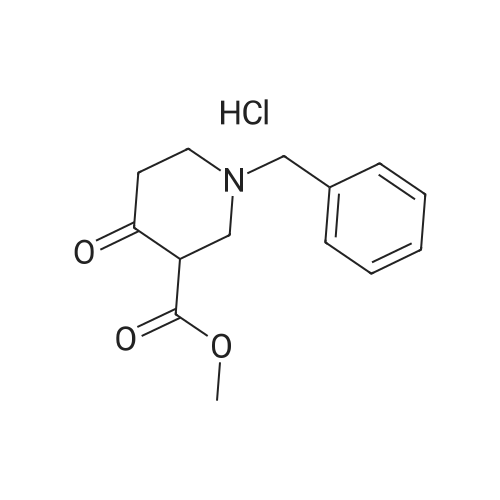
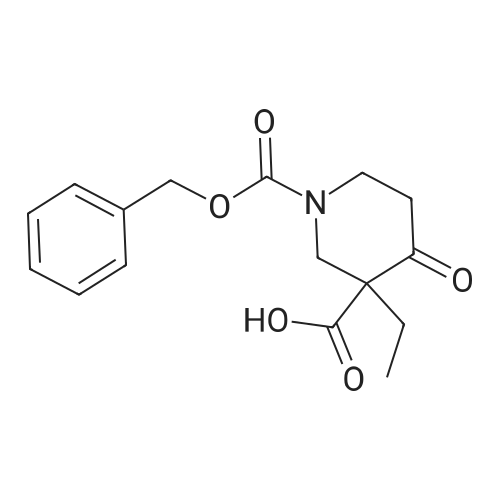
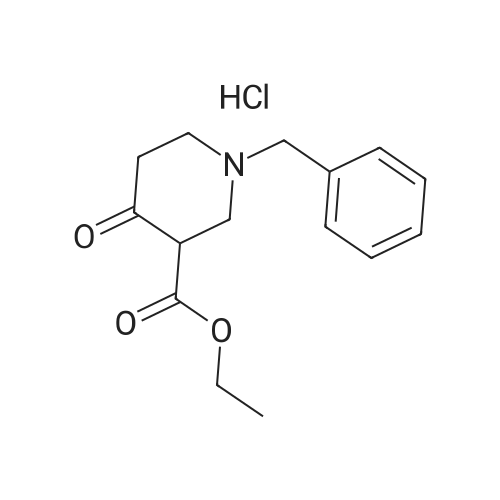
6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one A mixture of ethyl 1-benzyl-4-oxopiperidine-3-carboxylate hydrochloride (50.0 g, 0.168 mol), formamidine acetate (16.2 g, 0.201 mol), 4.37 M of sodium methoxide in methanol (190 mL) and methanol (200 mL, 5 mol) was heated to 85 C. for 16 hour in a 350 ml sealed reaction vessel. The mixture was allowed to cool and reduced in vacuo. The residue was dissolved in 1N NaOH (150 ml) and poured over ice. Glacial acetic acid was added to the mixture until the pH of the mixture was 7 and a tan solid precipitated out. the solid was filtered, washed with water and cold ether, and dried on high vacuum to yield the title compound as a tan solid. (26.2 g, 61.4%). MS: M+H=242.2. 1H NMR (DMSO-d6): delta 2.29 (t, 5.8 Hz, 2H); 2.61 (t, 5.8 Hz, 2H); 3.26 (s, 2H); 3.64 (s, 2H); 7.21-7.36 (m, 6H); 7.96 (s, 1H). |
| 61.4% |
|
Intermediate 16-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one A mixture of ethyl 1-benzyl-4-oxopiperidine-3-carboxylate hydrochloride (50.0 g, 0.168 mol), formamidine acetate (16.2 g, 0.201 mol), 4.37 M of sodium methoxide in methanol (190 mL) and methanol (200 mL) was heated at 85 C. for 16 hour in a 350 mL sealed reaction vessel. The mixture was allowed to cool and concentrated in vacuo. The residue was dissolved in 1N NaOH (150 mL) and poured over ice. Glacial acetic acid was added to the mixture until the pH of the mixture was 7 and a tan solid precipitated out. The solid was filtered, washed with water and cold ether, and dried on high vacuum to yield the title compound as a tan solid (26.2 g, 61.4%).LC-MS: 242.2 [M+1]+; 1H NMR (400 MHz, DMSO-d6): delta 2.29 (t, 2H, J=5.8 Hz), 2.61 (t, 2H, J=5.8 Hz), 3.26 (s, 2H), 3.64 (s, 2H), 7.21-7.36 (m, 6H), 7.96 (s, 1H). |
| 61.4% |
|
INTERMEDIATE 1 6-Benzyl-5,6,7,8-tetrahydropyrido [4,3-d] pyrimidin-4(3H)-one[00273] A mixture of ethyl l-benzyl-4-oxopiperidine-3-carboxylate hydrochloride (50.0 g,0.168 mol), formamidine acetate (16.2 g, 0.201 mol), 4.37 M of sodium methoxide in methanol (190 mL) and methanol (200 mL) was heated at 85 0C for 16 hour in a 350 mL sealed reaction vessel. The mixture was allowed to cool and concentrated in vacuo. The residue was dissolved in IN NaOH (150 mL) and poured over ice. Glacial acetic acid was added to the mixture until the pH of the mixture was 7 and a tan solid precipitated out. The solid was filtered, washed with water and cold ether, and dried on high vacuum to yield the title compound as a tan solid (26.2 g, 61.4 %).MS: 242.2 [M+l]+; 1H NMR (400 MHz, DMSO-d6): 2.29 (t, 2H, J = 5.8 Hz), 2.61 (t, 2H, J = 5.8 Hz), 3.26 (s, 2H), 3.64 (s, 2H), 7.21-7.36 (m, 6H), 7.96 (s, IH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping