|
In N,N-dimethyl-formamide; at 20℃; |
A solution of 20a, sodium methoxide (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford 20b. |
|
In N,N-dimethyl-formamide; at 20℃; |
A solution of R-27a (CAS Reg. No. 1435-51-4), MeONa (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford R-27b. |
|
In N,N-dimethyl-formamide; at 20℃; |
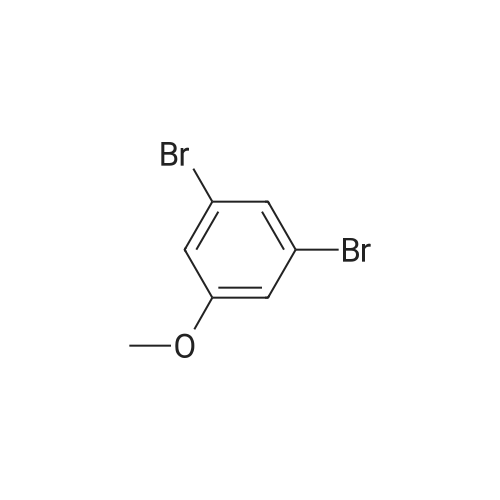
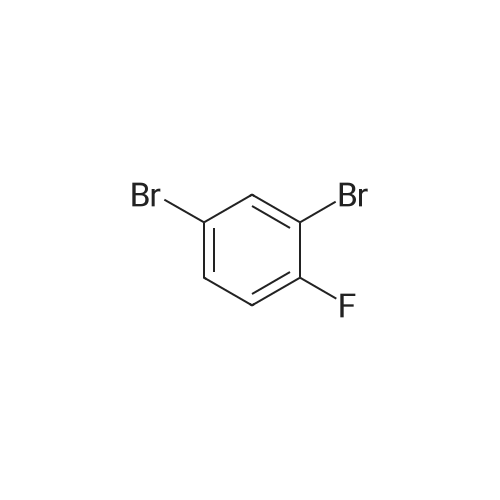
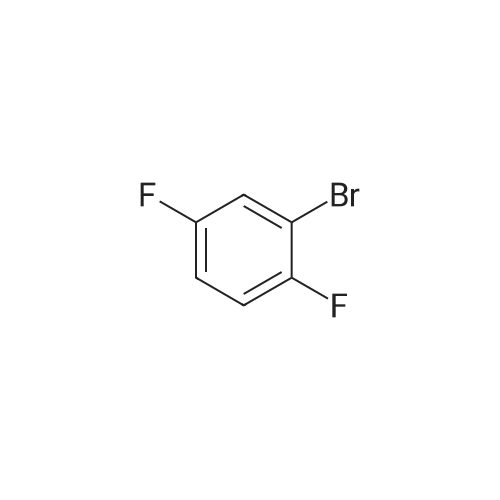
step 1-A solution of 1,3-dibromo-5-fluoro-benzene (CASRN 1435-514), MeONa (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford 1,3-dibromo-5-methoxy-benzene. |
|
In N,N-dimethyl-formamide; at 20℃; |
A solution of 1,3-dibromo-5-fluoro-benzene (CASRN 1435-51-4), MeONa (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford 1,3-dibromo-5-methoxy-benzene. |
|
In N,N-dimethyl-formamide; at 20℃; |
step 1-A solution of R-27a (CASRN 1435-51-4), MeONa (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford R-27b. |
|
In methanol; DMF (N,N-dimethyl-formamide); at 0 - 20℃; for 1h; |
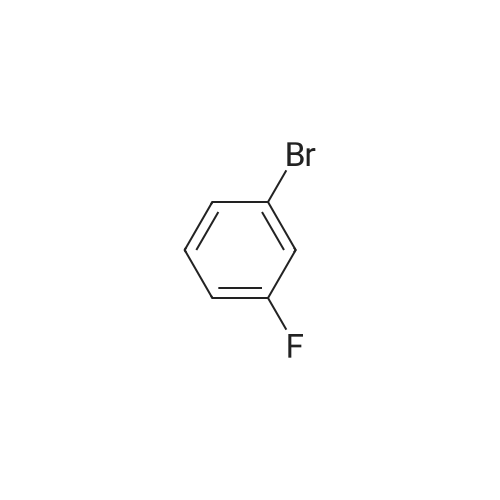
Sodium methoxide (8. 80ml of a 4.5M solution in methanol, 39. 6mmol) was added dropwise to a stirred solution of 3, 5-dibromofluorobenzene [(5.] [00G,] [19.] 0mmol) [(ALDRICH)] in N,N-dimethylformamide (95ml) at [0C] under nitrogen. The reaction was allowed to warm to room temperature, stirred for 1 hour and then concentrated under reduced pressure. The residue was dissolved in ether (500ml) and the resulting solution was washed with water [(3X300ML)] and brine (300ml), dried over magnesium sulphate, filtered and concentrated under reduced pressure to provide the title compound (5.13g) as a white solid. [H-NMR (300MHZ, CDCI3) ]: 8 = 3.79 (s, 3H), 7.00 (s, 2H), 7.26 (s, 1H). LRMS (thermospray): [M/Z] [[MH+]] 266. Microanalysis : Found: C, 31.56 ; H, 2.29. [C7H60BR2] requires C, 31.62 ; H, 2.27percent. |
|
In methanol; DMF (N,N-dimethyl-formamide); at 0 - 20℃; for 1h; |
Preparation 2 1 3-Dibromo-5-methoxybenzene Sodium methoxide (4.5M solution in methanol, 8.80 [ML,] 41.0 [MMOL)] was added dropwise to a stirred solution of 3, 5-dibromofluorobenzene (5.00 g, 19.0 [MMOL)] in N, N-dimethylformamide (95 ml) at [0°C] under a nitrogen atmosphere. The reaction was warmed to room temperature, stirred for 1 hour and then evaporated under reduced pressure. The residue was dissolved in diethyl ether and was washed with water [(3X300] [ML)] and brine (300 ml), dried over magnesium sulphate, filtered and concentrated under reduced pressure to give the title compound as a white solid (5.13 g).[H-NMR] [(300MHZ,] CDC13) : [8] 3.79 (s, 3H), 7.00 (s, 2H), 7.26 (s, [1 H).] LRMS: m/z TS+ 266 [[M+H] +.] |
|
In DMF (N,N-dimethyl-formamide); at 0 - 20℃; for 1h; |
Sodium methoxide (4.5M solution in methanol, 8.80 [ML,] 41.0 [MMOL)] was added dropwise to a stirred solution of 3, 5-dibromofluorobenzene (5.00 g, 19.0 [MMOL)] in [N, N-DIMETHYLFORMAMIDE] (95 ml) at [0°C] under a nitrogen atmosphere. The reaction was warmed to room temperature, stirred for 1 hour and then evaporated under reduced pressure. The residue was dissolved in diethyl ether and was washed with water [(3X300] [ML)] and brine (300 [ML),] dried over magnesium sulphate, filtered and concentrated under reduced pressure to give the title compound as a white solid (5.13 g). 'H-NMR [(300MHZ,] CDC13) : [8] 3.79 (s, 3H), 7.00 (s, 2H), 7.26 (s, [1 H).] LRMS: m/z TS+ 266 [[M+H] +.] |
|
In methanol; N,N-dimethyl-formamide; at 0 - 20℃; for 1h; |
Sodium methoxide (8.80 ml of a 4.5M solution in methanol, 39.6 mmol) was added dropwise to a stirred solution of 3,5-dibromofluorobenzene (5.00 g, 19.0 mmol) in N,N-dimethylformamide (95 ml) at 0° C. under nitrogen. The reaction was allowed to warm to room temperature, stirred for 1 hour and then concentrated under reduced pressure. The residue was dissolved in ether (500 ml) and the resulting solution was washed with water (3.x.300 ml) and brine (300 ml), dried over magnesium sulphate, filtered and concentrated under reduced pressure to provide the title compound (5.13 g) as a white solid. 1H-NMR (300 MHz, CDCl3): delta=3.79 (s, 3H), 7.00 (s, 2H), 7.26 (s, 1H). LRMS (thermospray): m/z [MH+] 266. Microanalysis: Found: C, 31.56; H, 2.29. C7H6OBr2 requires C, 31.62; H, 2.27percent. |
|
In DMF (N,N-dimethyl-formamide); at 20℃; |
step 1-A solution of 57a, sodium methoxide (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford 57b. |
|
In N,N-dimethyl-formamide; at 20℃; |
Preparation of 3-cyano-5-difluoromethyl-phenol step 8-; A solution of 10a, sodium methoxide (1 equivalent) and DMF were stirred overnight under an N2 atmosphere at RT. The volatile solvents were removed in vacuo and the residue partitioned between Et2O and water. The organic phase was washed with 5percent NaOH, water and brine, dried (MgSO4), filtered and evaporated to afford 10b. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping