| 86.9% |
With N-ethyl-N,N-diisopropylamine; HATU; In N,N-dimethyl-formamide; at 20℃; for 0.25h;Inert atmosphere; |
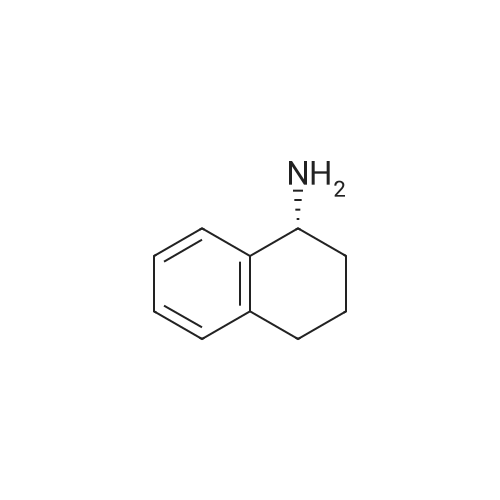
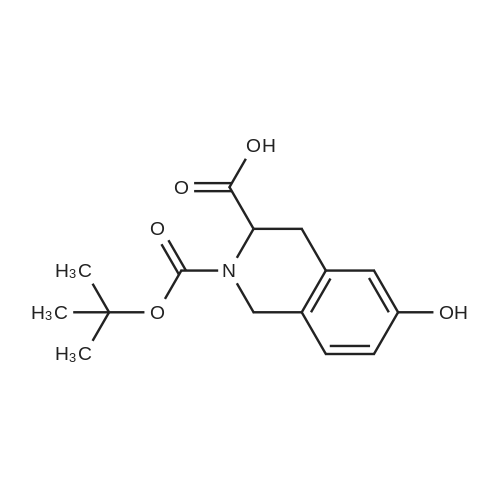
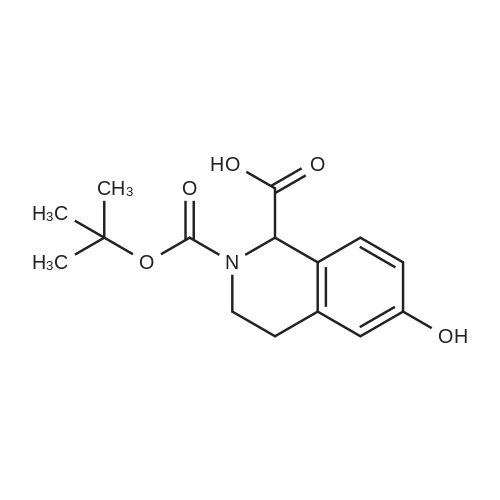
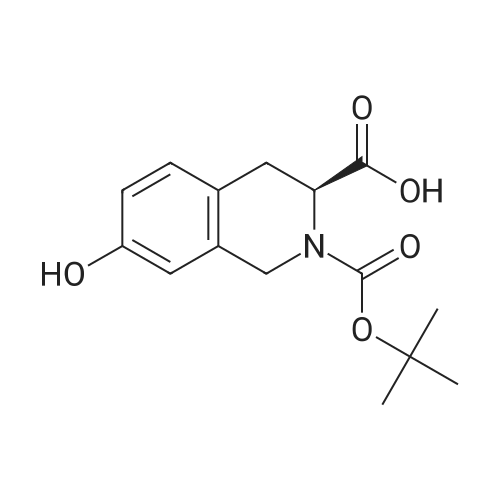
To a mixture of (S)-2-(tert-butoxycarbonyl)-7-hydroxy- 1,2,3,4- tetrahydroisoquinoline -3- carboxylic acid (32 g, 109 mmol) and (R)-1,2,3,4- tetrahydronaphthalen-1-amine (19.3 g, 131 mmol, CAS 23357-46-2) in DMF (150 mL) was added HATU (54 g, 142 mmol) and DIPEA (42 g, 328 mmol), and the mixture was stirred at rt for 15 min. The solution was then poured into water(1500 mL) and extracted with EtOAc (200 mL x 2). The combined organic layer was washed with brine (500 mL x 3), dried over Na2SO4, filtered, concentrated in vacuo and purified via column chromatography (Petroleum ether / EtOAc= 4 / 1) to give the title compound (40.2 g, 86.9 % yield) as a white solid. LC-MS (ESI+): m/z 423.1 (M+H)+ |
| 82% |
With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In N,N-dimethyl-formamide; at 20℃; for 0.5h; |
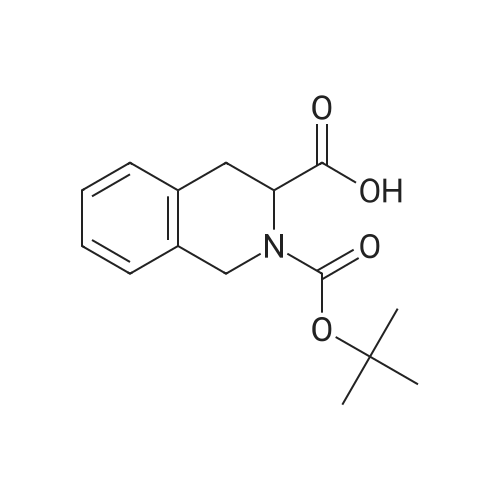
A mixture of (S)-2-(tert-butoxycarbonyl)-7-hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (commercially available from, for example, Fluorochem) (1 g, 3.41 mmol) and(R)-1,2,3,4-tetrahydronaphthalen-1-amine (commercially available from, for example, Aldrich) (0.552 g, 3.75 mmol) in DMF (4 mL) was treated with DIPEA (1.79 mL, 10.2 mmol) and then with HATU (1.56 g, 4.09 mmol) and stirred at ambient temperature for 30 minutes. The mixture was treated with dichloromethane (60 mL), saturated aqueous sodiumbicarbonate (10 mL) and water (10 mL) and separated through a hydrophobic frit. The organic phase was evaporated to dryness and the product was purified by chromatography on silica using a gradient elution from 0% to 100% ethyl acetate in cyclohexane to afford the title compound (1.18 g, 2.79 mmol, 82 % yield). LCMS RT= 1.10 mi ES-i-ye 423. |
| 82% |
With N-ethyl-N,N-diisopropylamine; HATU; In N,N-dimethyl-formamide; at 20℃; for 0.5h; |
(0116) A mixture of (S)-2-(tert-butoxycarbonyl)-7-hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (commercially available from, for example, Fluorochem) (1 g, 3.41 mmol) and (R)-1,2,3,4-tetrahydronaphthalen-1-amine (commercially available from, for example, Aldrich) (0.552 g, 3.8 mmol) in DMF (4 mL) was treated with DIPEA (1.8 mL, 10.2 mmol) and then with HATU (1.56 g, 4.1 mmol) and stirred at ambient temperature for 30 minutes. The mixture was treated with dichloromethane (60 mL), saturated aqueous sodium bicarbonate (10 mL) and water (10 mL) and separated through a hydrophobic frit. The organic phase was evaporated to dryness and the product was purified by chromatography on silica using a gradient elution from 0% to 100% ethyl acetate in cyclohexane to afford the title compound (1.18 g, 2.8 mmol, 82% yield). LCMS RT=1.10 min, ES+ve 423. |
| 1.25 g |
|
General procedure: To a 0 C solution of (5)-2-(tert-butoxycarbonyl)-7-hydroxy-l,2,3,4- tetrahydroisoquinoline-3-carboxylic acid (1.32 g, 4.49 mmol) in DMF (30 mL) was added EDC (1.03 g, 5.39 mmol) followed by HOAt (0.73 g, 5.39 mmol). After 10 min, (R)- 1,2,3,4-tetrahydronaphthalen-l -amine (Alfa Aesar, 0.66 mL, 4.49 mmol) and NMM (1.48 mL, 13.5 mmol) were added. The resulting reaction mixture was allowed to warm to room temperature over 4 h and then poured into a separatory funnel containing EtOAc and sat. aq. NaHC03 solution. The aqueous layer was extracted with EtOAc (3x), and thecombined organic extracts were washed with IN HC1, 10% LiCl and sat. NaCl and then dried over MgS04, filtered and concentrated in vacuo. The crude oil was then purified by flash chromatography (gradient elution from 0 to 70% EtOAc/hexanes) to afford the title compound (1.25 g, 2.96 mmol, 66% over 3 steps) as a light yellow foam. XH NMR (CDC13) δ 7.18 - 6.86 (m, 4H), 6.84 - 6.67 (m, 1H), 6.58 - 6.39 (m, 1H), 6.04 - 5.74 (m, 2H), 4.94 (d, J = 5.7 Hz, 1H), 4.78 - 4.61 (m, 1H), 4.56 - 4.41 (m, 1H), 4.20 (d, J= 15.6 Hz, 1H), 3.34 (d, J= 13.4 Hz, 1H), 3.02 (d, J = 12.1 Hz, 1H), 2.81 - 2.60 (m, 2H), 1.87 - 1.68 (m, 2H), 1.67 - 1.54 (m, 2H), 1.53 - 1.37 (m, 9H); MS(ESI+) m/z 423.2 (M+H)+ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping