|
|
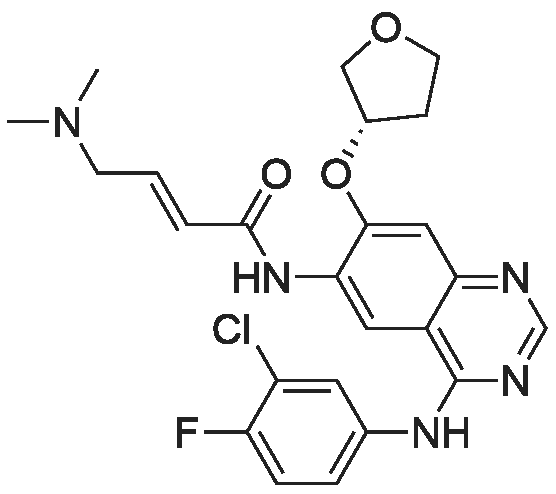
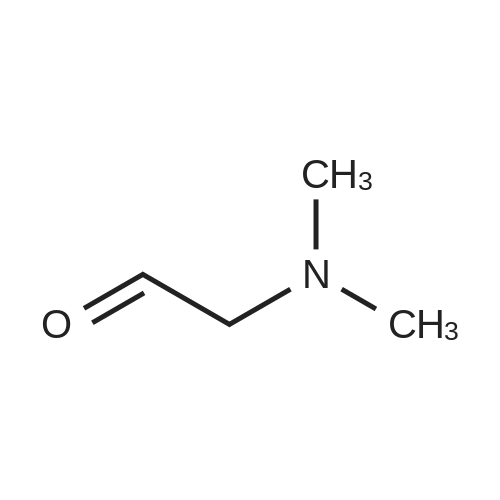
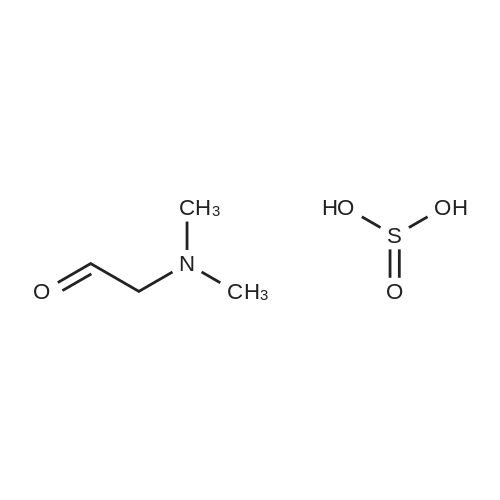
Starting material: 2-(dimethylamino)-acetaldehyde sulfite was prepared according to the method in WO2007/85638. [0193] Diethyl (2-((4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)amino)-1-fluoro-2-oxoethyl)phosphonate (1 eq.) and NaH (1.5 eq.) were dissolved in DMF (10 ml), and stirred at 0° C. for 30 min, then 2-(dimethylamino)-acetaldehyde sulfite (2.0 eq.) was added, ice bath was removed, and then the mixture warmed to room temperature naturally, and the reaction was stirred for another 3-5 h. After the reaction finished, the mixture was quenched with saturated NaHCO3, extracted with EtOAc, and the organic phase was dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, the crude product was purified by column chromatography (mobile phase 30:1 DCM/MeOH) and pale yellow of N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobut-2-enamide was given. MS (ESI+): m/z=448, 449, 450 [M+H]+. [0194] Rf values: 0.30 (Silica gel, ethyl acetate/methanol=5:1; two isomers were not separated); [0195] 0.53, 0.56 (Silica gel, dichloromethane/methanol=10:1; two isomers were separated). Example 2 [0196] The preparation of (Z)?N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobut-2-enamide and (E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-4-(dimethylamino)-2-fluorobut-2-enamide [0197] Use Gilson 215 semi-preparative chromatography (322 pump, 156 UV detector) to separate the mixture of cis and trans-isomers obtained in example 1. [0198] Column: Phenomenex Gimini 30×250 mm, 10 mum; [0199] Detection wavelength: 254 nm; [0200] Column temperature: room temperature; [0201] Sample treatment method: sample (a mixture of cis and trans isomers) was dissolved in methanol, filtered. Concentration: 22 mg/ml, the volume of each needle injection was 800 muL. [0202] Mobile phase: water:acetonitrile (with 0.05percent aqueous ammonia)=49:51 [TABLE-US-00001] Time (min) Water (percent) Acetonitrile(percent) Gradient: 0 49 51 15.0 29 71 15.5 0 100 18.0 0 100 18.5 49 51 20.0 49 51 [0203] Collected the component at retention time of 14.5 min to obtain (Z)-isomer (Compound 2-1); and retention time of 16.0 min to obtain (E)-isomer (Compound 2-2). [0204] (Z)-Isomer Hydrochloride [0205] The obtained (Z)-isomer was dissolved in ethyl acetate, and concentrated hydrochloric acid was added dropwise until pH=1.0 to precipitate solid, a little diethyl ether was added dropwise, and the mixture was stirred overnight at room temperature, filtered and the residue was dried under reduced pressure to obtain hydrochloride. [0206] 1H NMR [(CD3)2SO]: delta 11.41 (s, 1H), 10.30 (s, 1H), 8.99 (s, 1H), 8.88 (s, 1H), 8.04 (dd, 1H), 7.77-7.70 (m, 1H), 7.58 (s, 1H), 7.56 (dd, 1H), 6.47 (td, 1H), 4.03 (s, 3H), 3.99 (br, 2H), 2.79 (s, 6H). [0207] (E)-Isomer [0208] 1H NMR (300 MHz, DMSO) delta 10.26 (s, 1H), 10.02 (s, 1H), 8.92 (d, J=2.7 Hz, 1H), 8.88 (d, J=1.5 Hz, 1H), 8.01 (dd, J1=6.6 Hz, J2=2.1 Hz, 1H), 7.70-7.67 (m, 1H), 7.56 (t, J=9.0 Hz, 1H), 7.42 (d, J=2.7 Hz, 1H), 6.34 (dt, J1=33.6 Hz, J2=7.5 Hz, 1H), 4.06 (s, 3H), 4.04 (brs, 2H), 2.33 (s, 6H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping