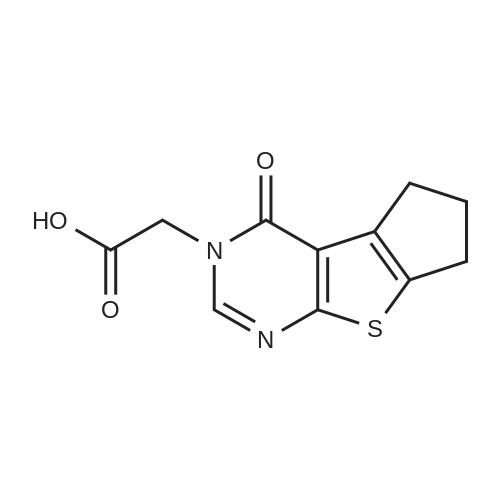
| 87% |
With bromine; acetic acid; at 80℃; for 3.0h; |
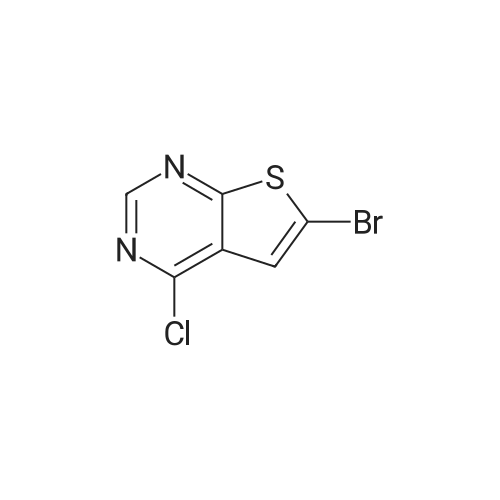
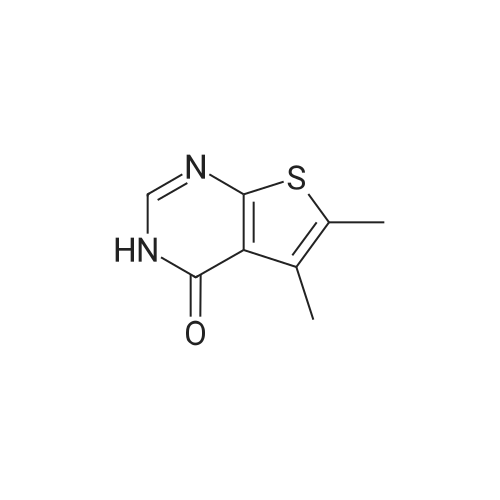
Thieno[2,3-d]pyrimidin-4(3H)-one (2) (25.1 g, 165 mmol) was mixed with concd acetic acid (425 mL), and bromine (17.0 mL, 52.8 g, 330 mmol) was added slowly before the mixture was heated at 80 C for 3 h. The reaction mixture was then cooled to rt and filtered to remove insoluble components. The liquid fraction was diluted with ice and neutralised using a saturated aq NaHCO3 solution. The precipitated material was isolated by filtration and washed with water (6×100 mL). Drying gave 33.1 g (143 mmol, 87%) of 3 as a light brown solid, mp. 283-290 C (dec), (lit.40 301-304 C). A fraction of this material was purified before it was used in Suzuki coupling. The dry material was applied to the top of a packed silica gel plug and eluted with CH2Cl2, mp. 298-301 C; 1H NMR (400 MHz, DMSO-d6) delta: 12.63 (s br, 1H), 8.14 (s, 1H), 7.55 (s, 1H); 13C NMR (100 MHz, DMSO-d6) delta: 165.0, 156.0, 146.5, 125.6, 124.6, 110.3; IR (neat, cm-1): 3074, 1663, 1649, 1504, 755; HRMS (EI, 70 eV, m/z): 229.9146 (calcd C6H3Br79N2OS, 229.9144, M+). The 1H NMR spectrum corresponds with Ref. 41. 13C NMR spectroscopy data has not previously been reported. |
| 56% |
With bromine; potassium acetate; In acetic acid; at 0 - 20℃; for 24.0h; |
[00113] To a stirred solution of thieno[2,3-d]pyrimidin-4(3H)-one (4.0 g, 12.33 mmol) and potassium acetate (17.25 g, 210.32 mmol) in acetic acid (100 mL) was added slowly bromine (2.7 mL, 52.57 mmol) at 0 C. The reaction mixture was stirred at room temperature for 24 h. After completion of the reaction, the reaction mixture was concentrated, the residue was basified with saturated sodium bicarbonate solution and extracted with ethyl acetate (2 x 1000 mL).The combined organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to afford the title compound 6-bromothieno[2,3-d]pyrimidin-4(3H)-one (3.4 g crude, 56% yield) as brown solid. [00114] Calculated (M+H): 230.91; Found (M+H): 231.0. |
|
With bromine; acetic acid; at 20℃; for 4.0h; |
A suspension of 3flr-thieno[2,3-
|
|
With bromine; In dichloromethane; acetic acid; |
EXAMPLE 15 6-Bromo-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (Compound 6g) The product from Example 14 (0.75 g) was added to glacial acetic acid (10 ml) and heated with stirring until it dissolved. Bromine (0.75 ml) was then added and the mixture immediately set solid. More acetic acid was added and the mixture broken up. It was then heated at 80 C. for 61/2 hours, cooled and poured into ice-water. The solid was filtered and washed with water followed by dichloromethane and dried to give the title product, m.p. 304 C. |
|
With bromine; acetic acid; at 20℃; for 16.0h; |
The mixture of 60.1 g 3H-thieno[2,3-djpyrimidin-4-one (0.395 mol), 605 mL acetic acidand 24 mL bromine (0.468 mol) was stirred at room temperature for 16 hours. The reactionmixture was monitored by LCMS. Further bromine was added in three portions (12 mE, 5 mE, 10 mE) until the conversion exceeded 95%. The precipitate was filtered off, washed with acetic acid (3x50 mE), diethyl ether (3x100 mL) and then air dried to give the product as a tan powder. |
| 4.76 g |
With bromine; acetic acid; at 80℃; for 5.0h;Inert atmosphere; |
Under argon atmosphere, 2.50 mL of bromine was slowly added to 3.66 g of acetic acid solution (62.0 mL) of thieno [2,3-d] pyrimidin-4 (3H) -one with stirring and stirred at 80 C. for 5 hours, The temperature was returned to room temperature. The precipitated solid was separated by filter paper filtration, washed with acetic acid and water in this order, neutralized with saturated aqueous layer water, and then washed with water. After drying under reduced pressure, 4.76 g of the title compound was obtained as a brown solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping