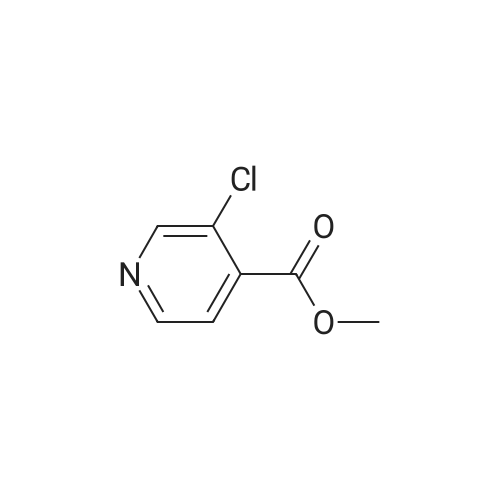
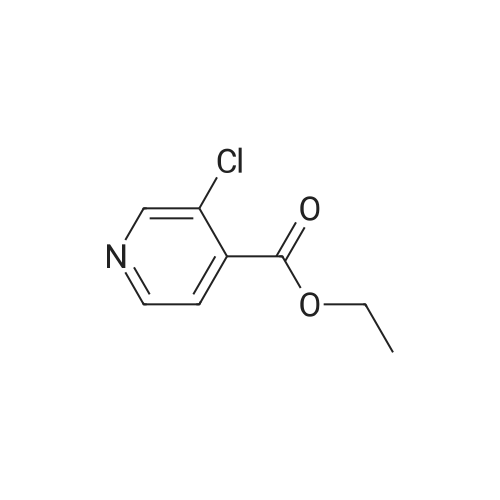
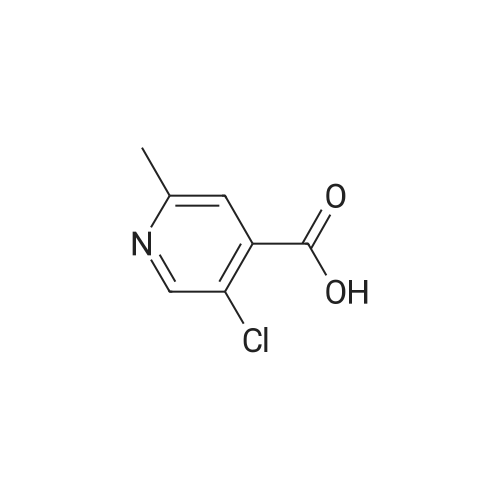
|
With thionyl chloride; In N-methyl-acetamide; 1,2-dichloro-ethane; |
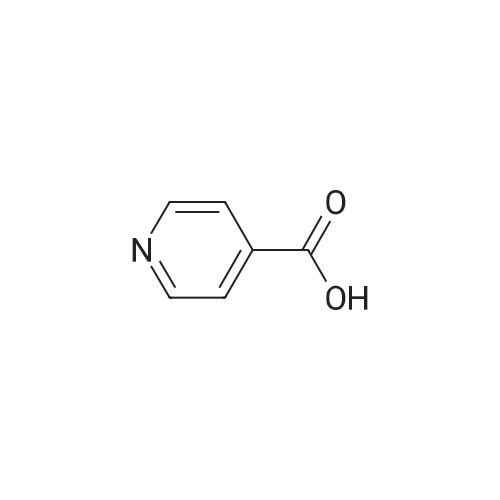
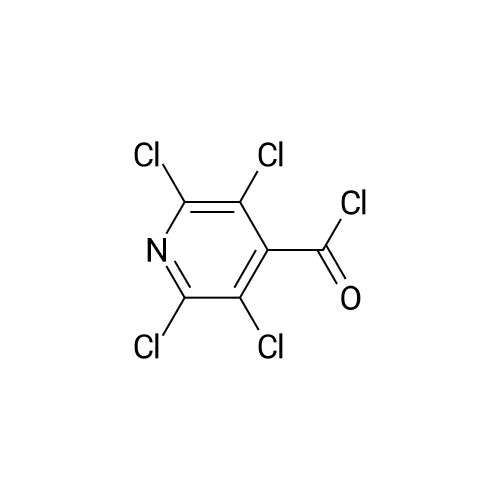
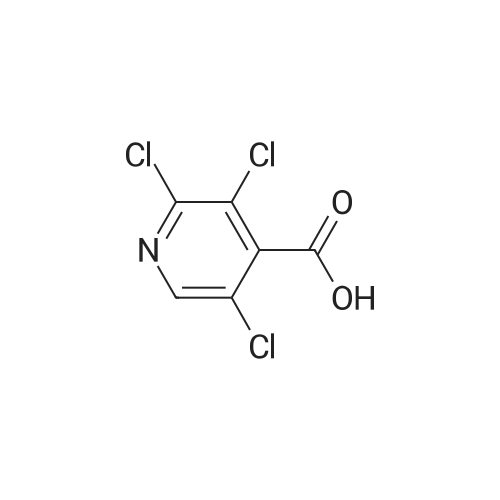
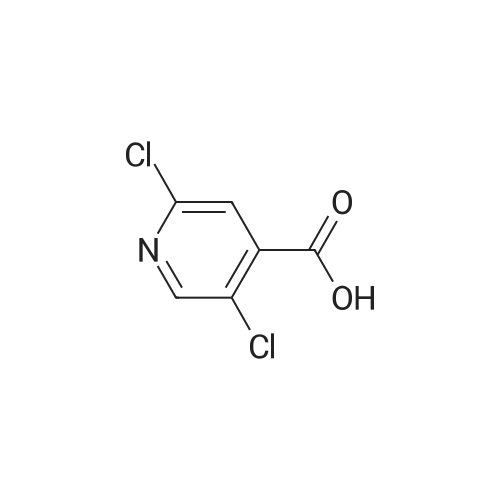
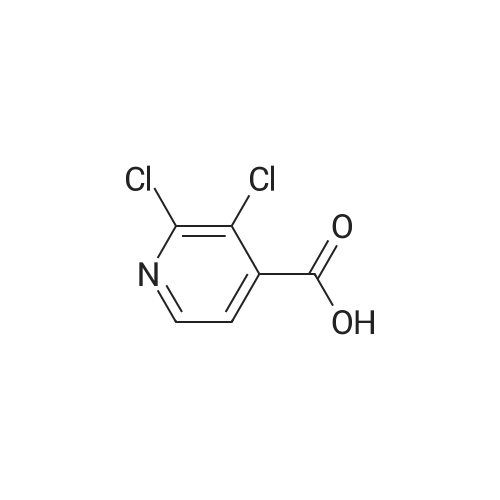
Thus obtained <strong>[13958-93-5]3,5-dichloropyridine-4-carboxylic acid</strong> (384 mg, 2 mmol) was dispersed in 1,2-dichloroethane(30 mL) to prepare a slurry, added with thionyl chloride (0.44 mL, 5 mmol), further added with dimethylformamide (0.3 mL), and the mixture was refluxed under heating for 1 hour. The solvent was evaporated under reduced pressure to thereby obtain a crude product of 3,5-dichloropyridine-4-carbonylchloride. |
|
With thionyl chloride; In DMF (N,N-dimethyl-formamide); dichloromethane; for 4h;Heating / reflux; |
Intermediate 2 (S)-Ethyl-3-[4-(3.5-dichloropyrid-4-ylcarboxamido)phenyl]-2-(t-butoxycarbonylamino)propionate A slurry of Intermediate 1 (51.2 g, 0.267 mol) in DCM (195 ml) and thionyl chloride (195 ml, 2.67 mol) was treated with DMF (5 drops) and heated to reflux for 4 h.. The reaction was concentrated in vacuo and azeotroped with toluene (2*50ml) to give the acid chloride derivative of intermediate 1 as a yellow solid which was used without further purification. |
|
With thionyl chloride; N,N-dimethyl-formamide; In dichloromethane; for 4h;Heating / reflux; |
A slurry of the compound of Intermediate 1 (51.2g, 0. 267mol) in DCM [(195MUT)] and thionyl chloride [(195MOI,] 2. [67MOL)] was treated with DMF (5 drops) and heated to reflux for 4h. The reaction was concentrated in vacuo and azeotroped with toluene [(2X50M1)] to give a yellow solid which was used without further purification. A solution of [ETHYL- (S)-3- (4-] aminophenyl)-2- (t-butoxycarbonylamino) propanoate (130.8g, 0. [425MOL)] in [DCM (800ML) WAS COOLED TO 0XB0; AND TREATED WITH NMM 0.51MOL),] stirred for 5 minutes and then a solution of the acid chloride (98.3g, 0. [468MOL)] in DCM [(200ML)] was added dropwise keeping the reaction temperature [BELOW 5XB0;.] The reaction was stirred for 1h, quenched with NaHCO3 solution [(500ML),] the organic layer separated, washed with NaHCO3 [SOLUTION (500ML),] 10% citric acid solution [(500MOI)] and [NAHCO3] [SOLUTION (500M1),] dried [(MGS04)] and concentrated in vacuo to give a yellow solid which was [RECRYSTALLISED] (EtOAc/hexane) to give the title compound, (140g, [69%).] [8H] (DMSO d6), 8.8 (2H, s), 7.55 (2H, d, J 8.5Hz), 7.23 (2H, d, J 8.5Hz), 4.0 (3H, m), 3.4 (2H, b s), 2.9 (1H, m), 2.8 [(1 H,] m), 1.3 (9H, s), 1.25 (3H, t); m/z (ES+, 70V) 504 (MNa+). |
|
With thionyl chloride;N,N-dimethyl-formamide; In dichloromethane; for 3h;Heating / reflux; |
Preparation of the 3,5-dichloro-isonicotinoyl chloride, The acid (1.50 g, 7.81 mmol) was placed in the round-bottom flask equipped with stirrer and reflux condenser, DCM (20 ml_) containg 2 drops of DMF was added as one portion followed by thionyl chloride (1.40 g, 0.85 ml, 1.5 eq.). The reaction mixture was refluxed for 3 hr resulting clear solution. The solution was evaporated in vacuum providing yellow oil, which was used in the next step without purification. |
|
With thionyl chloride; for 7h;Reflux; |
Reference Production Example 11 A mixture of 1 g of <strong>[13958-93-5]3,5-dichloroisonicotinic acid</strong> and 5 ml of thionyl chloride was heated to reflux for seven hours. Then, the mixture was cooled to room temperature, and then concentrated under reduced pressure. The residue was dissolved in 3 ml of DMF, which was added dropwise to a mixture of 2-amino-4-trifluoromethylphenol, 5 ml of DMF and 1.05 g of triethylamine at 0C. The reaction mixture was stirred at room temperature for two hours, and then water was added thereto, followed by extraction with ethyl acetate twice. The combined organic layers were washed with water and a saturated sodium chloride solution, dried over magnesium sulfate, and concentrated under reduced pressure. The residue was washed with diethyl ether to give 0.75 g of 3,5-dichloro-N-(2-hydroxy-5-trifluoromethylphenyl)isonicotinamide. [Show Image] 1H-NMR (CDCl3+DMSO-d6) delta: 9.03 (br s, 1H), 8.59 (s, 2H), 8.45 (d, J=2.0 Hz, 1H), 7.30 (dd, J=8.5, 2.2 Hz, 1H), 7.04 (d, J=8.5 Hz, 1H) |
|
With thionyl chloride; for 7h;Reflux; |
Reference Production Example 11A mixture of 1 g of <strong>[13958-93-5]3,5-dichloroisonicotinic acid</strong> and 5 ml of thionyl chloride was heated to reflux for seven hours. Then, the mixture was cooled to room temperature, and then concentrated under reduced pressure. The residue was dissolved in 3 ml of DMF, which was added dropwise to a mixture of 2-amino-4-trifluoromethylphenol, 5 ml of DMF and 1.05 g of triethylamine at 0 C. The reaction mixture was stirred at room temperature for two hours, and then water was added thereto, followed by extraction with ethyl acetate twice. The combined organic layers were washed with water and a saturated sodium chloride solution, dried over magnesium sulfate, and concentrated under reduced pressure. The residue was washed with diethyl ether to give 0.75 g of 3,5-dichloro-N-(2-hydroxy-5-trifluoromethylphenyl)isonicotinamide.1H-NMR (CDCl3+DMSO-d6) delta: 9.03 (br s, 1H), 8.59 (s, 2H), 8.45 (d, J=2.0 Hz, 1H), 7.30 (dd, J=8.5, 2.2 Hz, 1H), 7.04 (d, J=8.5 Hz, 1H) |
|
With thionyl chloride; N,N-dimethyl-formamide; In dichloromethane; at 40℃; for 2.5h; |
To a suspension of <strong>[13958-93-5]3,5-dichloroisonicotinic acid</strong> (52 mmol) in 250 mL DCM and 5 mL DMF were added 1 1.4 mL thionylchloride. The mixture was heated to 40C for 2.5 h, cooled to RT before 100 mL EtOH were added. The solution was stirred at RT for 10 min and then concentrated in vacuo. The crude mixture was taken up in EtOAc and quenched with sat. aq. NaHC03 solution under ice cooling. The aqueous layer was extracted with EtOAc (2x). The combined organic layers were washed with brine, dried over MgS04 and concentrated in vacuo. Purification by CC using Hept/EtOAc (95/5 to 85/15) gives the desired product as colorless oil; |
|
With thionyl chloride; In dichloromethane; N,N-dimethyl-formamide; for 3h;Reflux; |
To a solution of KR-i (1.50 g, 7.85 mmol) in DCM (20 mL), containing two drops of DMF, was added thionyl chloride (1 .40 g, 0.85 mL, 1 .5 eq). The reaction was refluxed for 3 hours resulting a clear solution. The solution was evaporated in vaccum providing the crude product KR-4 (1 .35 g, 82.3% yield) as a yellow oil. ESI-MS (Mi-i): 210 calc. for C6H2CI3NO: 208.9. |
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 20℃; for 3h; |
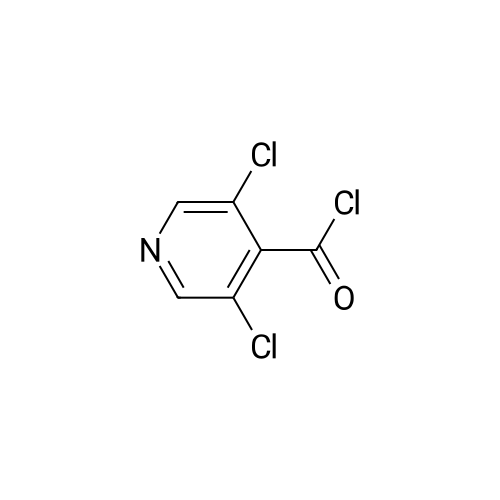
i) To a suspension of <strong>[13958-93-5]3,5-dichloroisonicotinic acid</strong> (MANCHESTER, 50 g, 260.42 mmol) inDCM (500 ml) was added oxalyl chloride (ALDRICH, 24.24 ml, 286.462 mmol) and 30 dropsof N,N-Dimethylformamide (DMF).The reaction mixture was stirred at rt for 3h. UPLC with NHMe2 in CH3CN showed reaction was completed.The solvent was evaporated in vacuo to yield the desired product as a greenish solid (3,5-dichloropyridine-4-carbonyl chloride) and was used for the next step without any further purification. |
|
With thionyl chloride;N,N-dimethyl-formamide; In dichloromethane; for 5.75h;Heating / reflux; |
To a nitrogen flushed 500 mL round bottom flask was charged with 3,5-dichloro- isonicotinic acid (46.5 g, 0.24 mol), CH2CI2 (150 mL), DMF (0.5 mL), and thionyl chloride (20 mL,33.9 g 0.28 mol). After the slurry was refluxed for 5 h, additional thionyl chloride (5 mL, 0.70 mol) and CH2CI2 (100 mL) were added, and the reaction was refluxed for additional 45 min. The reaction mixture was concentrated, and the residue was azeotroped with toluene to give the crude acyl chloride, which was used immediately. Thus, the crude acyl chloride was dissolved in CH2CI2 (150 mL) and was added to N-BOC-4-amino-L-phenylalanine ethyl ester (60 g, 0.20 mol) and 4-methylmorpholine (44 mL, EPO <DP n="20"/>0.40 mol) in CH2CI2 (400 mL) at O0C over 5 min. After stirring at 00C for 1 h, the reaction was quenched with dilute aqueous NaHCtheta3. The organic layer was separated and the aqueous layer was extracted with CH2CI2 (500 mL). The organic layers were combined, dried over anhydrous MgSO4 and concentrated in vacuo, and the residue was purified by flash column chromatography on silica gel eluting with 4:1 to 3:2 EtOAc/hexanes to afford the title compound (95 g, 100% yield). lH NMR (400 MHz, CD3OD) delta 8.60 (s, 2H), 7.54 (d, 2H), 7.20 (d, 2H), 4.20-4.36 (m, IH), 4.10 (q, 2H), 3.02-3.12 (m, IH)5 ),2.82-2.92 (in, IH), 1.34/1.30 (s, 9H).1.20 (t, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping