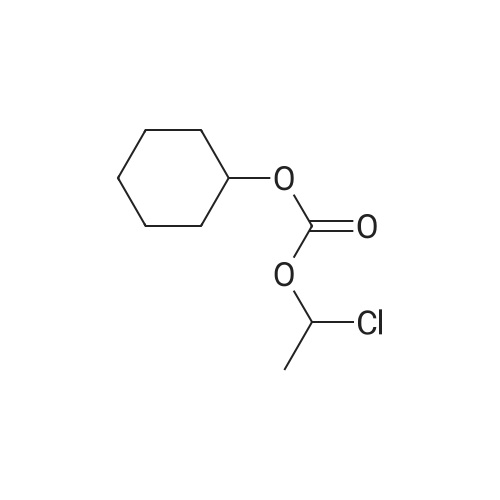
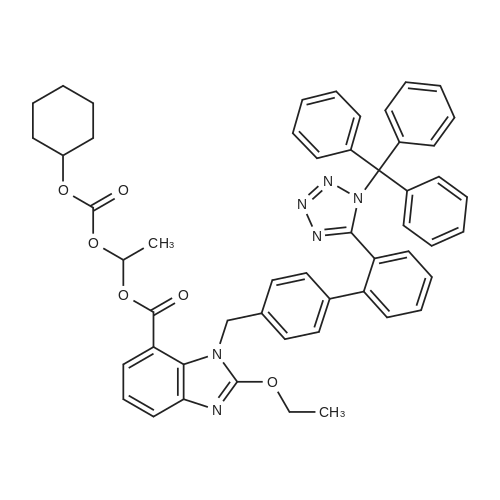
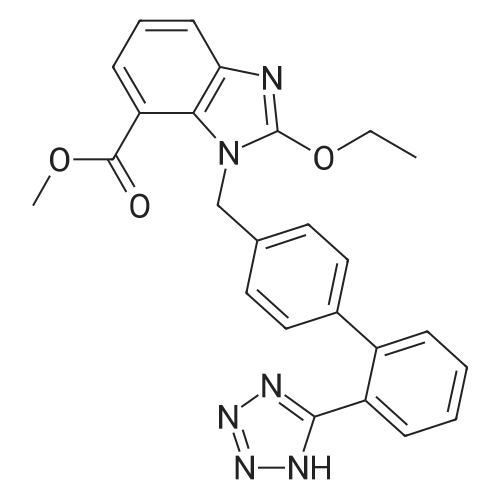
| 95% |
With potassium carbonate; In N,N-dimethyl-formamide; at 55 - 60℃; for 3h; |
A mixture of trityl Candesartan, dimethylformamide (DMF) and potassium carbonate at was heated at 60-70 C. Cyclohexyl 1-chloroethylcarbonate was added at 55-60 C. to the reaction mixture and maintain for 3 hours at 55-60 C. The reaction mixture was cooled at ambient temperature. The reaction mixture was poured in water at 0-10 C. and stirred for one hour at 0-10 C. The mixture was filtered and washed with D. M. water. A mixture of wet cake and acetone was stirred and heated for 30 minutes at 55-60 C. The reaction mixture was cooled and stirred at ambient temperature for 30 minutes. The mixture was filtered and washed with acetone. The solid was dried to obtain tritylated Candesartan cilexetil.Yield: 92-95% |
| 92 - 95% |
With potassium carbonate; In N,N-dimethyl-formamide; at 55 - 70℃; for 3h; |
Exaniples-4; Preparation of tritylated Candesartan cilexetilA mixture of trityl Candesartan, dimethylformamide (DMF) and potassium carbonate at was heated at 60-700C. Cyclohexyl 1-chloroethylcarbonate was added at 55-600C to the reaction mixture and maintain for 3 hours at 55-600C. The reaction mixture was cooled at ambient <n="16"/>temperature. The reaction mixture was poured in water at 0-100C and stirred for one hour at 0-100C. The mixture was filtered and washed with D. M. water. A mixture of wet cake and acetone was stirred and heated for 30 minutes at 55-600C. The reaction mixture was cooled and stirred at ambient temperature for 30 minutes. The mixture was filtered and washed with acetone. The solid was dried to obtain tritylated Candesartan cilexetil. Yield: 92-95 % |
| 84.8% |
With potassium carbonate; In acetonitrile; at 40℃; for 8h;Product distribution / selectivity; |
EXAMPLES; Example 1: Method of Making Cilexetil Trityl Candesartan in a Low Boiling Solvent; A suspension of trityl candesartan (2.0 g, 2.93 mmol), cilexetil chloride (1.21 g, 5.86 mmol), potassium carbonate (0.81 g, 5.86 mmol) and acetonitrile (19 g) was stirred at 40C for about 8 h, and the reaction was monitored by TLC. The acetonitrile was removed at 30C to 35 C under reduced pressure (10 mbar), and the residue was mixed with water (20 ml) and ethyl acetate (30 ml). The water layer was separated and extracted with ethyl acetate (20 ml x 2). The combined organic layers were washed with brine (10 ml x 2), dried over sodium sulfate, and evaporated to give cilexetil trityl candesartan crude, as a semi-solid, 94.38 % pure by HPLC. The crude product was triturated with hexane (30 ml) at 25C to 27C for about 3 h. Thereafter, the solids were filtered off, washed on the filter with hexane (5 g x 2) and dried at 25 C to 27C under reduced pressure (10 mbar) to give cilexetil trityl candesartan (12 g, 84.8 %) 94.64% pure by HPLC. |
| 67.2% |
With tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate; In toluene; at 50 - 55℃; for 8.5h;Product distribution / selectivity; |
Example 2: Method of Making Cilexetil Trityl Candesartan with a PTC; A suspension of trityl candesartan (2.0 g, 2.93 mmol), cilexetil chloride (1.21 g, 5.86 mmol), potassium carbonate (1.22 g, 8.83 mmol), and tetrabutylammoniumhydrogensulfate (0.2 g) in toluene (20 ml) was stirred at 50C to 55 C for about 8. 5 h. The reaction progress was monitored by TLC. The mixture was poured into water (100 ml) and neutralized with citric acid (solid). The organic layer was separated, washed with water, and extracted with ethyl acetate (20 ml x 3). The combined organic layers were washed with brine (10 ml), dried over sodium sulfate, and evaporated. The residue was triturated with hexane (20 ml) at 20-25C for about 30 min, filtered and dried at 40C and at less than about 30mbar to give white powder (1.68 gr, 67.2%), with 97.90% purity by HPLC. |
|
With potassium carbonate; potassium iodide; In N,N-dimethyl-formamide; at 25 - 65℃; |
13,6 kg candesartan is dissolved 43,3 kg DMF at temperature bellow 25C; thereto add 4,1 kg Threeethylamine and 10,4 kg trityl chloride and heat up to 60-650C. After the reaction has completed the reaction mixture is poured into ethanol preheated to 50 +/-2C and thereto water is added. Upon cooling pH is adjusted with aqueous HCI to 4.6. Isolated tritylcandesartan is dissolved in 50kg DMF, and mixed at 25C; whereupon 2,2 kg potassium iodide, 4,4 kg potassium carbonate and 6,6 kg cilexetil chloride are added and mixture is heated to 60-65 0C until the reaction is completed. The product is isolated. |
|
With potassium carbonate; potassium iodide; In dimethyl sulfoxide; at 60 - 65℃; for 2.5h;Product distribution / selectivity; |
Step-2: Preparation of Trityl candesartan cilexetil; Carbohexyl 1-chloroethyl carbonate (36 g) is added to a suspension of trityl candesartan (100 g), potassium carbonate (24 g) and potassium iodide (12 g) in DMSO (500 ml) at temperature of 60 - 65C over 30 min. Reaction mass is maintained at 60-650C for 2 hrs, added toluene (300 ml) and water (300 ml). Reaction mass is mixed for 15 min., allowed to settle, the layers are separated at 60 - 650C and aqueous layer is extracted with toluene (200 ml). Water (200 ml) washings are given to the combined organic layer and toluene extractions twice at temperature of 60 - 650C. Toluene is distilled off from water washed organic layer at temperature below 6O0C under vacuum, ethanol (100 ml) is added, mixed for about 30 min and distilled off solvents under vacuum at temperature below 600C under vacuum. Residue is cooled to 30 - 35C, ethanol (300 ml) is added, mixed for 2 hrs at 25 - 300C and filtered the product. Wet cake is washed with ethanol (100 ml) and suck dried. Wet weight of Cilexetil trityl candesartan is 180 g; Example 2: Preparation of Candesartan cilexetil (without isolation of cilexetil trityl candesartan); Carbohexyl 1-chloroethyl carbonate (36 g) is added to a suspension of trityl candesartan (100 g), potassium carbonate (24 g) and potassium iodide (12 g) in DMSO (500 ml) at <n="8"/>temperature of 60 - 65C over 30 min. Reaction mass is maintained at 60-650C for 2 hrs, added toluene (300 ml) and water (300 ml). Reaction mass is mixed for 15 min., allowed to settle, the layers are separated at 60 - 650C and aqueous layer is extracted with toluene (200 ml). Water (200 ml) washings are given to the combined organic layer and toluene extractions twice at temperature of 60 - 650C. Toluene is distilled off from water washed organic layer at temperature below 6O0C under vacuum, ethanol (100 ml) is added, mixed for about 30 min and distilled off solvents under vacuum at temperature below 6O0C under vacuum. Residue is cooled to 30 - 350C, ethanol (1000ml) and boric acid (9.0 g) is added at temperature of 25 - 300C, temperature of reaction mass is raised and maintained at reflux temperature for 8 hrs. Reaction mass is concentrated to one third of its original volume by distillation of solvent and cooled the solution to 25 - 3O0C.n-Hexane (500 ml) is added to the reaction mass, mixed for 8hrs at 25 - 3O0C and filtered the product. Wet cake is washed with n-hexane (100 ml) and dried the material at temperature of 45-500C till becomes constant weight. Dry weight of Cilexetil candesartan is 65 g (Yield: 72.5%) |
|
With potassium carbonate; potassium iodide; In N,N-dimethyl-formamide; at 20 - 75℃; |
Potassium carbonate (60 gm), 1-chloroethylcyclohexyl carbonate (60 gm) and potassium iodide (20 gm) were added to the solution of 2-Ethoxy-1-[[2'-(N- triphenylmethyltetrazole-5-yl)biphenyl)-4-yl]methyl]benzimidazole-7-carboxylic acid (100 gm) in dimethylformamide (500 ml) at room temperature. Raised the temperature to 750C, stirred for 2 hours, cooled to room temperature and 5% sodium chloride solution (2000 ml) was added. Maintained for 15 minutes, ethyl acetate (400 ml) was added, stirred and separated the layers. Aqueous layer was extracted with ethyl acetate (400 ml), organic layer was taken, washed with 10% sodium chloride solution (400 ml), concentrated, and co-distilled with ethyl acetate (100 ml). Mixture of ethyl acetate (500 ml) and n-hexane (500 ml) were added to the residual mass, stirred for 6 hours at room temperature, cooled to 50C, stirred for 1 hour, filtered, then washed with mixture of ethyl acetate (50 ml) and n-hexane (200 ml) and dried for 6 hours to obtain 1-(Cyclohexyloxy <n="6"/>carbonyloxy)ethyl-2-ethoxy-1-[[2'-(1H-tetrazole-5-yl)biphenyl-4-yl]methyl] benzimidazole-7-carboxylate (110 gm, HPLC Purity: 99%). |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 - 65℃; for 2.5h; |
Step I: Preparation of N-trityl candesartan cilexetilA mixture of N-trityl candesartan (Form A: 120 gm), potassium carbonate (48.58 gm), cilexetil chloride (54.48 gm) and dimethyl formamide (180 mL) at ambient temperature was heated to 60 C to 65 C followed by stirring at the same temperature for 2 hours and 30 minutes. The reaction mixture was cooled to 25 C to 30 C followed by addition of dichloromethane (600 mL) and ice cooled de-ionized water (1200 mL) at 10 C to 15 C to the reaction mass. The reaction was further stirred at 15 C to 20 C for 30 minutes followed by extraction of the aqueous layer with dichloromethane (120 mL) at 15 C to 30 C and washing with de-ionized water (2x600 mL) at ambient temperature.The organic layer was concentrated completely under vacuum at 30 C to 35 C followed by removal of traces of dichloromethane with cyclohexane (120 mL) and the further addition of cyclohexane (360 mL) to the residue at the ambient temperature. The reaction mixture was stirred at the same temperature for 14 hours followed by filtration and washing of the solid with cyclohexane (120 mL) which was suck dried under vacuum for 1 hour. Dichloromethane was again added (360 mL) at ambient temperature to the isolated solid followed by heating and stirring of the reaction mass at 30 C to 35 C for 30 minutes. The organic layer was concentrated under vacuum at 30 C to 35 C, cyclohexane (300 mL) added and the reaction mass was stirred further for 5 hours at the same temperature. The solid was filtered, washed with cyclohexane (120 mL) and suck dried under vacuum for 1 hour followed by further drying under vacuum for 16 hours at 35 C to 40 C.Chromatographic purity: 98.8%N-trityl desethylcandesartan cilexetil- 0.28% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +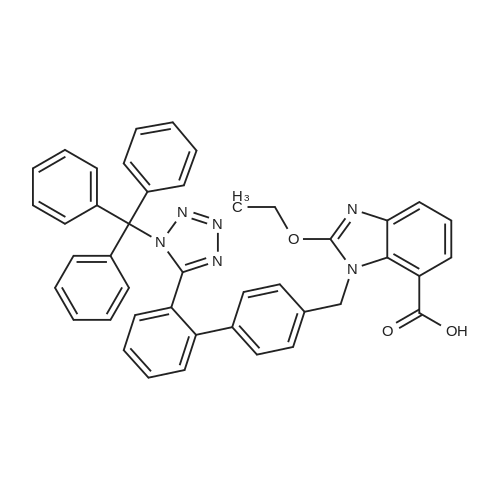

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping