| 67% |
With indium(III) chloride; sodium tetrahydroborate; In tetrahydrofuran; at 25℃; for 16h;Inert atmosphere; |
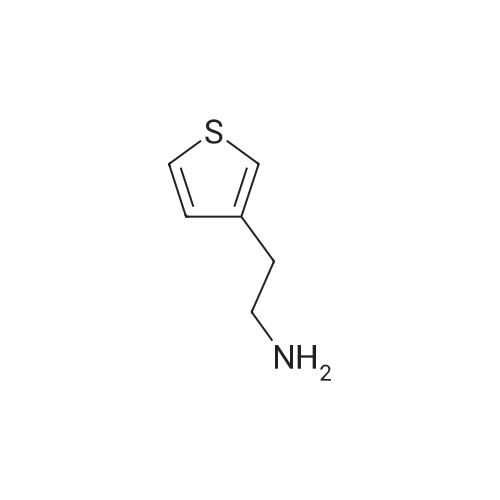
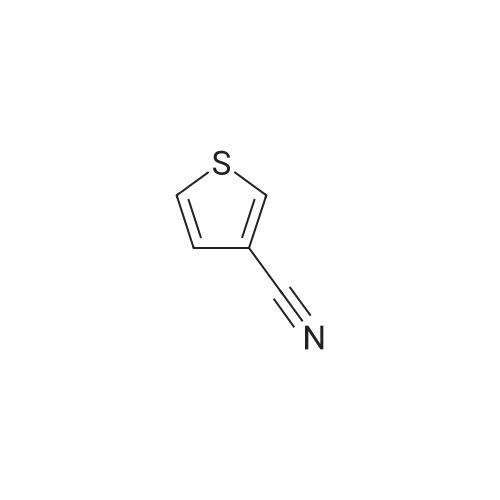
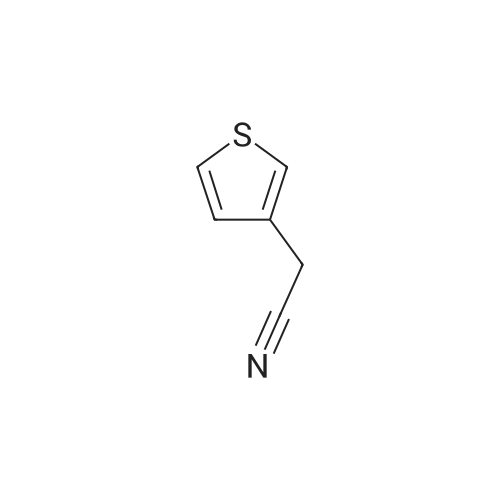
This compound was synthesized according to a procedure described in literature.5 An oven-dried round-bottom flask (25 mL) cooled under argon was fitted with a rubber septum and charged with a stir bar, anhydrous InCl3 (0.681 g, 3.079 mmol), anhydrous THF (10 mL), and NaBH4 (0.341 g, 9.014 mmol). The reaction was stirred at 25 C for 1h, followed by the dropwise addition of 3-thiopheneacetonitrile (0.340 mL, 2.981 mmol), and the mixture was stirred at 25 C for 16 h. The solution was quenched with 10 mL of 2.8 M hydrochloric acid, and the solution was refluxed for 2 h to dissolve remaining metal salts. The reaction mixture was cooled to 25C, 5 mL of methanol were added, and the mixture was again refluxed for 2 h. The reaction mixture was then cooled to 25C and filtered, and the methyl borate/methanol was removed from the filtrate by evaporation. The remaining acidic solution was extracted with DCM (3 10 mL), and the organic layers were discarded. The acidic aqueous layer was then basified with NaOH pellets to pH ≈ 11 and again extracted with DCM (3 10 mL). The combined organic layers were dried on a WA filter filtered, and evaporated under vacuum to afford 2-(3-thienyl)ethanamine 7. Yellowish oil; 0.250 g, yield: 67%. 1H NMR (300 MHz, CDCl3): δ = 7.20-7.30 (m, 1H; ArH), 7.00 (m, 1H; ArH), 6.95 (m, 1H; ArH), 2.96 (t, 2H; J = 6.6 Hz, CH2), 2.78 (t, 2H; J = 6.6 Hz, CH2), 1.45 ppm (bs, 2H; NH2). The 1H NMR spectrum was according to literature. |
|
With hydrogenchloride;aluminium chloride; In diethyl ether; water; |
(a) Thiophene-3-ethylamine A solution of anhydrous aluminium chloride (26.6 g) in diethylether (100 ml) was added to a stirred suspension of lithium aluminium hydride (7.6 g) in ether (100 ml) under nitrogen atmosphere at room temperature. To the stirred mixture was added thiophene-3-acetonitrile (24.6 g) in ether dropwise over 30 minutes, the exothermic reaction causing the mixture to reflux. After 1 hour water (8 ml) was added cautiously (exothermic) followed by 5M aqueous HCl (400 ml). The aqueous layer was separated and basified to pH~11 with 50% aqueous NaOH (160 ml). The aqueous solution was extracted with dichloromethane (2*200 ml) and the extracts dried over magnesium sulphate, filtered and evaporated to a pale yellow liquid. Distillation gave thiophene-3-ethylamine as a colourless liquid, b.p. 78-79 at 6 mmHg. |
|
With hydrogenchloride;aluminium chloride; In diethyl ether; water; |
(a) Thiophene-3-ethylamine A solution of anhydrous aluminium chloride (26.6 g) in diethylether (100 ml) was added to a stirred suspension of lithium aluminium hydride (7.6 g) in ether (100 ml) under nitrogen atmosphere at room temperature. To the stirred mixture was added thiophene-3-acetonitrile (24.6 g) in ether dropwise over 30 minutes, the exothermic reaction causing the mixture to reflux. After 1 hour water (8 ml) was added cautiously (exothermic) followed by 5M aqueous hydrochloric acid (400 ml). The aqueous layer was separate and basified to pH ~11 with 50% aqueous sodium hydroxide (160 ml). The aqueous solution was extracted with dichloromethane (2*200 ml) and the extracts dried over magnesium sulphate, filtered and evaporated to a pale yellow liquid (25.0 g). Distillation gave thiophene-3-ethylamine as a colourless liquid, b.p. 78-79 at 6 mmHg. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping