|
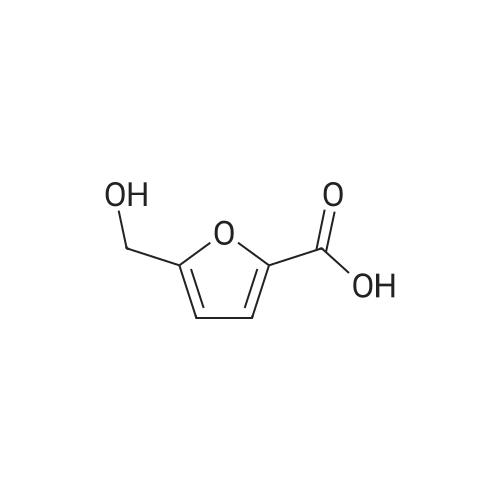
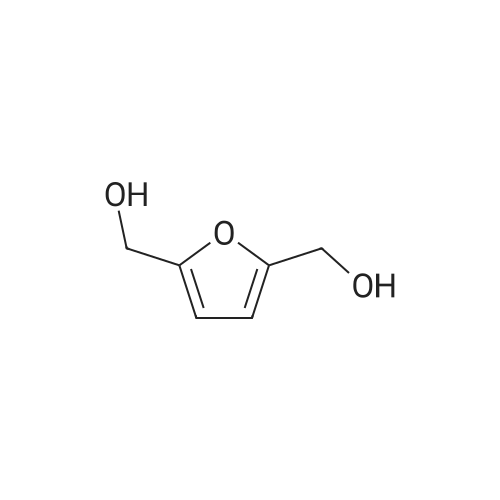
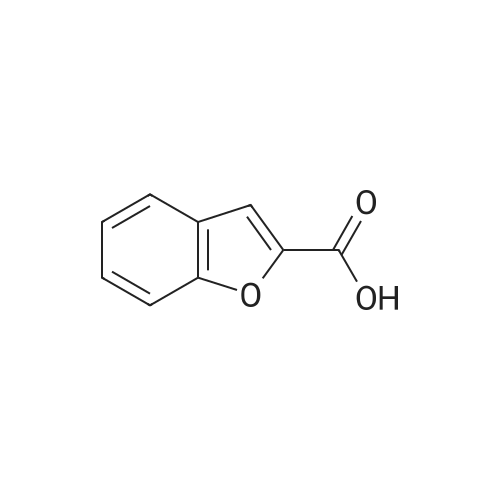
With platinum on carbon; water-d2; oxygen; at 100℃; under 75007.5 Torr; for 4h;Autoclave; |
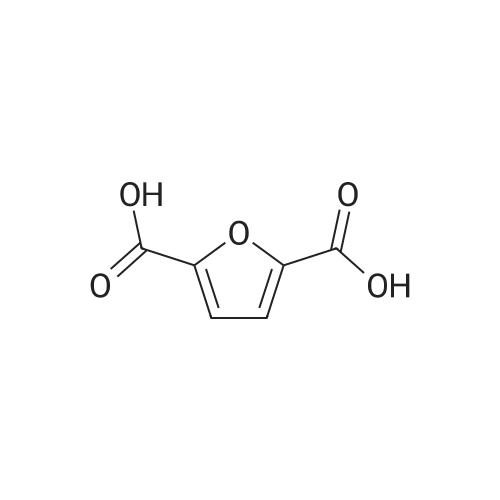
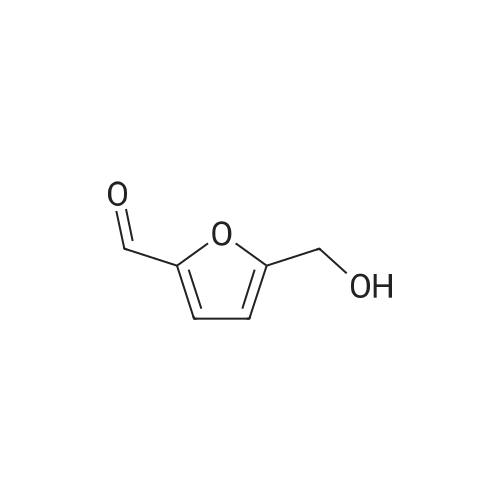
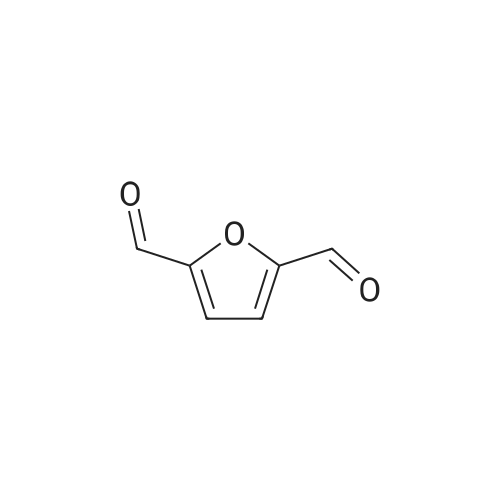
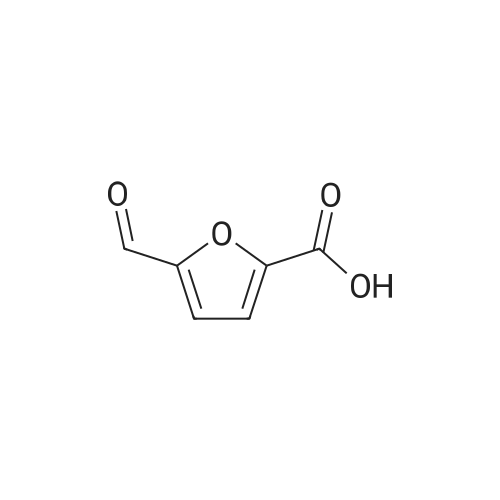
l-b Catalyst screening experiments: Catalyst screening was carried out in a series of single experiments designated "Screen 1 " to "Screen 7". In each single experiment "Screen 1 " to "Screen 7" the organic reactant compound HMF (compound of Formula (II)) was in parts catalytically converted by means of at least one heterogeneous platinum catalyst (see Tables 1 and 2, below) into FDCA (compound of formula (I)). The general experimental procedure for each screening experiment of "Screen 1 " to "Screen 7" was as follows: In a first step, an aqueous reactant mixture was prepared by filling a specific amount of deuterated water (D20, 99,9 atom%, Sigma Aldrich (151882)) and a specific amount of HMF (99+%, Sigma Aldrich (W501808)) into a steel autoclave reactor (inner volume 60 ml or 90 ml, respectively, for exact information see Table 2, below). In case a steel autoclave reactor with an inner volume of 60 ml was used the amounts of HMF and D20 were as follows: D20: 18,0 g, HMF: 2,0 g (corresponding to 15,9 mmol as starting amount of HMF). In case a steel autoclave reactor with an inner volume of 90 ml was used the amounts of HMF and D20 were as follows: D20: 27,0 g, HMF: 3,0 g (corresponding to 23,8 mmol as starting amount of HMF). The starting concentration C0[HMF] of HMF in each aqueous reactant mixture was 10 % by weight, based on the total mass of the aqueous reactant mixture (total mass of deuterated water and HMF). The respective amount of solid heterogeneous catalyst as stated in Table 2 was added to the respective aqueous reactant mixture and, thus, a reaction mixture comprising deuterated water, HMF, and the heterogeneous catalyst was obtained. After adding the specific amount of heterogeneous catalyst the obtained reaction mixture appeared as a deep black slurry, the black color apparently caused by the black solid particles of the heterogeneous catalyst. The molar ratio of substrate to metal of the heterogeneous catalyst (HMF : Pt) was approximately 100 : 1. In a second step, the filled reactor was tightly sealed and pressurized with synthetic air (total pressure 100 bar, Oxygen (as part of the synthetic air) : HMF ratio is approximately 2,25 : 1 ) to obtain conditions for catalytic conversion. The present reaction mixture was heated to a temperature of 100C while stirring at 2000 rpm. After reaching 100C this temperature was maintained for 4 or 20 hours, respectively, (see Table 2 "Reaction time" for exact information) while continuing stirring the heated and pressurized reaction mixture during the reaction time. As a result, a first product suspension comprising FDCA in solid form and the heterogeneous catalyst in solid form was formed. |
|
With sodium carbonate; at 100℃; under 30003 Torr; for 0.33h;pH 9.10; |
Experimental Conditions The HMF having 95% purity was supplied by Interchim. The method of the invention was implemented in a discontinuous reactor under pressure in a 300 mL autoclave equipped with magnetically driven gas-inducing agitator. Heating was ensured by a heating collar connected to a PID controller (proportional integral derivative). A sampling gate allowed the taking of a sample from the reaction medium via an immersed tube, allowing the monitoring of the progress of the reaction over time. The samples were analysed by HPLC chromatography with two RID detectors (Refractive Index Detector) and PDA (Photodiode array) (ICE-Coragel 107H column, eluting with 10 mM H2SO4). Total Organic Carbon (TOC) in solution was also analysed using a TOC analyser and the value measured was compared with the mass balance (MB) calculated by HPLC. The reactor was charged with 150 mL of 100 mM aqueous HMF solution (2 g), a weight of catalyst corresponding to a HMF/Pt molar ratio of 100, and the desired amount of base in the form of NaOH (comparative example), NaHCO3, KHCO3, Na2CO3 or K2CO3 expressed as base/HMF molar ratio. Air was added at a pressure of 40 bar and the reactor heated to 100 C. The influence of the Bi/Pt ratio was also examined in the presence of Na2CO3 (Na2CO3/HMF=2). The same trend was observed when comparing a series of bimetallic catalysts having molar ratios varying between 0.07 and 1. The results are grouped together in Tables 11 to 15. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping