Alternatived Products of [ 135062-02-1 ]
Product Details of [ 135062-02-1 ]
| CAS No. : | 135062-02-1 |
MDL No. : | MFCD00906179 |
| Formula : |
C27H36N2O4
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | FAEKWTJYAYMJKF-QHCPKHFHSA-N |
| M.W : |
452.59
|
Pubchem ID : | 65981 |
| Synonyms : |
AG-EE 623ZW
|
Chemical Name : | (S)-2-Ethoxy-4-(2-((3-methyl-1-(2-(piperidin-1-yl)phenyl)butyl)amino)-2-oxoethyl)benzoic acid |
Safety of [ 135062-02-1 ]
Application In Synthesis of [ 135062-02-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 135062-02-1 ]
- 1
-
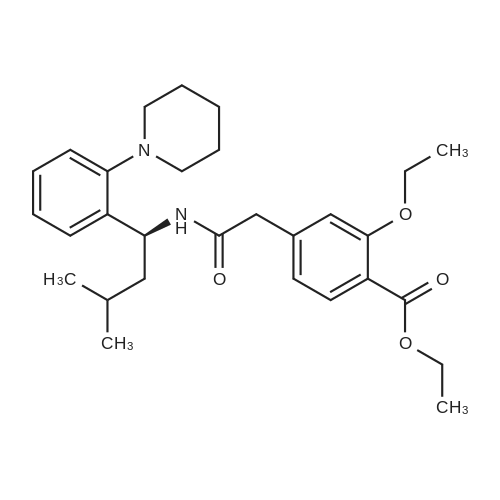 [ 147770-06-7 ]
[ 147770-06-7 ]

-
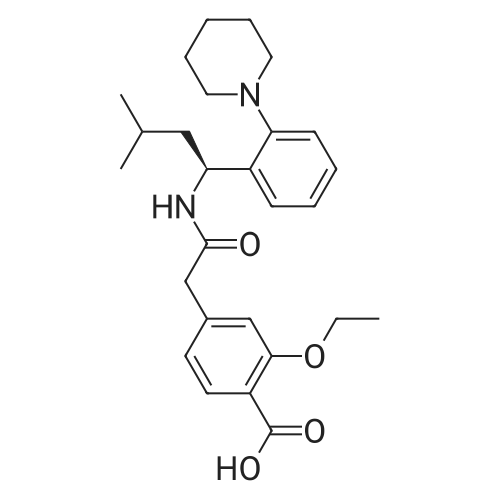 [ 135062-02-1 ]
[ 135062-02-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 87.6% |
With methanol; sodium hydroxide; for 3h;Reflux; |
Ethyl S-(+)-2-ethoxy-4-[N-{1-(2-piperidin-phenyl)-3-methyl-1-butyl} aminocarbonylmethyl]benzoate 119.4 g (0.248mol, 1eq) was dissolved in 280 g of methanol, 2.0 moL /L 248 mL of sodium hydroxide solution was added, heated to reflux for 3 hours, methanol was evaporated, cooled to room temperature, 2.0 moL / L hydrochloric acid was added to the reaction solution, the pH of the reaction solution was adjusted to 4-6, stirred for crystallization, filtered, washed with water and dried to give white crystalline repaglinide 98.3g, HPLC99.5%, 99.8% ee ,87.6% yield. |
| 83.2% |
|
EXAMPLE 3 Process for the Preparation of Repaglinide Ethyl (S)-2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-amino carbonylmethyl]-benzoate (4.5 g, 0.0093 mol) was dissolved in methanol (45 ml), followed by the addition of sodium hydroxide solution (0.75 g of sodium hydroxide dissolved in 6 ml water). The reaction mixture was heated at 60-65 C. for 3-4 hours. Methanol (80-85%) was removed from the reaction mixture under vacuum. The remaining reaction mixture was diluted with water (45 ml) and pH was adjusted to 6.5-7.0 with 1N HCl. The precipitated solid was stirred for 2-3 hours followed by filtration and washing with water (45 ml). The product was further dried at 50-55 C. under vacuum for 6-8 hours to produce Repaglinide (Yield=83.2%; HPLC Purity: 99.89%). |
| 83.2% |
|
Example 9Process for the preparation of 2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoic acid (Repaglinide)Ethyl(S)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonyl methyl]benzoate (4.5 g, 0.0093 moles) was dissolved in methanol (45 ml), which was followed by the addition of sodium hydroxide solution (0.75 g of sodium hydroxide dissolved in 6 ml water). The reaction mixture was heated at 60-65 C. for 3-4 hours. Methanol (80-85%) was removed from the reaction mixture under vacuum. The remaining reaction mixture was diluted with water (45 ml) and pH was adjusted to 6.5-7.0 with 1N HCl. The precipitated solid was stirred for 2-3 hours followed by filtration and washing with water (45 ml). The product was further dried at 50-55 C. under vacuum for 6-8 hours to produce repaglinide (Yield: 83.2%; HPLC purity: 99.81%; and Chiral purity by HPLC: 99.97%). |
| 81% |
|
To a solution of 2-ETHOXY-4-[3-METHYL-1-(2-PIPERIDIN-1-YL- PHENYL)-BUTYLCARBAMOYL]-METHYL}-BENZOIC acid ethyl ester (86 g, 0.18 mol) in ethanol (860 mL), a solution of sodium hydroxide (10.3 G, 0.26 mol) in water (260 mL) was added and stirred at 60-65 C for 2 h. Activated chrcoal (9 g) was added to the reaction mixture and filtered over celite bed. After adjusting the pH of the clear filtrate to 4.0-4. 2, the mixture was stirred at 40-45 C for 30 minutes. The mixture further cooled to 0-5 C and stirred for 1 h. The product was filtered and dried. Yield : 66 g, 81% |
|
|
EXAMPLE 5; PREPARATION OF REPAGLINIDE; 3-Ethoxy-4-(ethoxycarbonyl)benzene acetic acid (2.25 g, 0.0089 moles) was added to a solution of (alphaS)-alpha-(2-methylpropyl)-2-(1-piperidinyl)benzenemethanamine (2g, .008 moles) in toluene (20 ml). Then N,N-dicyclohexylcarbodiimide (1.95 g, 0.0094 moles) was added and the reaction mixture was stirred till completion of the reaction at 25-40C. The solid was filtered under suction and toluene filtrate was distilled under reduced pressure. The product was crystallized from ethanol / water and dried to yield <strong>[147770-06-7]ethyl 2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoate</strong> (3 g). 1N aqueous sodium hydroxide solution (8.0 ml) in ethanol (30 ml) was added to ethyl-2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoate (3 g) at 60-65C and stirred. After completion of the reaction the reaction mixture was cooled, pH was adjusted to 5 with 1N aqueous hydrochloric acid and product was filtered and dried at reduced pressure. The dried product was recrystallised from aqueous ethanol to give repaglinide. Yield: 2.27 g |
| 72% (28.5 g) |
|
Example 19 (S)(+)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonyl-methyl]-benzoic acid (Repaglinide) To a hot solution (40-45C) of crude <strong>[147770-06-7](S)(+)-2-ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonyl-methyl]-benzoic acid ethyl ester</strong> (35.7 g) in ethanol (320 ml), 112 ml of 1M NaOH (112 mmol) was added dropwise. After heating this mixture to a temperature of about 55-58C for 2.5h, charcoal was added for 15 minutes. Then, the hot suspension was filtrated. The filtrate was cooled to about 35C and 112 ml of 1M HCl (112mmol) was added slowly to give a suspension having a pH of about 5. After keeping this mixture at a temperature of about 20-25C for 2h and for another 2h at about 0-5C, the precipitated product was filtrated and washed with 30 ml of cooled ethanol 50%. The crude product was crystallized from a 2:1 mixture of EtOH and water (450 ml) and dried at 50C for 5h to obtain the desired (S)-repaglinide in a yield of 72% (28.5 g). |

Reference:
[1]Journal of Medicinal Chemistry,1998,vol. 41,p. 5219 - 5246
[2]Patent: CN109970681,2019,A .Location in patent: Paragraph 0043; 0053; 0054
[3]Asian Journal of Chemistry,2013,vol. 25,p. 9345 - 9350
[4]Patent: US2008/200684,2008,A1 .Location in patent: Page/Page column 4
[5]Patent: US2010/197732,2010,A1 .Location in patent: Page/Page column 13-14
[6]Patent: WO2004/103983,2004,A1 .Location in patent: Page 7; 8
[7]Chimia,2006,vol. 60,p. 593 - 597
[8]Patent: EP2019097,2009,A1 .Location in patent: Page/Page column 8
[9]Patent: WO2004/101540,2004,A2 .Location in patent: Page/Page column 13-14
[10]Patent: WO2004/101540,2004,A2 .Location in patent: Page/Page column 15-16
[11]Patent: EP2177221,2010,A1 .Location in patent: Page/Page column 15
- 2
-
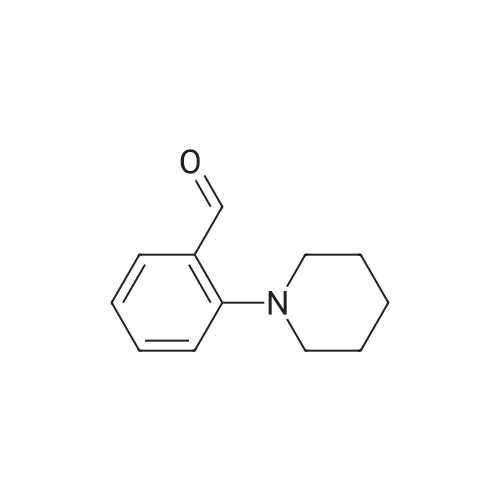 [ 34595-26-1 ]
[ 34595-26-1 ]

-
 [ 135062-02-1 ]
[ 135062-02-1 ]
Reference:
[1]Journal of Medicinal Chemistry,1998,vol. 41,p. 5219 - 5246
[2]Asian Journal of Chemistry,2013,vol. 25,p. 9345 - 9350
[3]Asian Journal of Chemistry,2013,vol. 25,p. 9345 - 9350
[4]Asian Journal of Chemistry,2013,vol. 25,p. 9345 - 9350
[5]Patent: CN109970681,2019,A
[6]Patent: CN109970681,2019,A
[7]Patent: CN109970681,2019,A
- 3
-
1sodium hydroxide
[ No CAS ]

-
 [ 147770-06-7 ]
[ 147770-06-7 ]

-
 [ 135062-02-1 ]
[ 135062-02-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In methanol; ethanol; |
EXAMPLE 9 (S)(+)-2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonylmethyl]-benzoic acid *0.4 H2 O Prepared from 89 mg (0.198 mMol) of ethyl (S)(+)-2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonylmethyl]-benzoate [melting point: 122-124 C.; [alpha]D20 =+8.3 (c=1 in methanol)] by saponification with 1sodium hydroxide solution in ethanol analogously to Example 3. Yield: 44.5 mg (48.8% of theory), Melting point: 102-103 C. (toluene/petroleum ether) Calc.: (*0.4 H2 O) C 70.51; H 8.01. Found: C 70.80; H 8.06. |
- 4
-
1N-hydrochloric acid
[ No CAS ]

-
 [ 147770-06-7 ]
[ 147770-06-7 ]

-
 [ 135062-02-1 ]
[ 135062-02-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In sodium hydroxide; ethanol; |
EXAMPLE 3 (S)-2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonylmethyl]-benzoic acid A solution of 3.79 g (7.88 mMol) of ethyl (S)-2-ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)aminocarbonylmethyl]-benzoate (ee =99.9%) in 37 ml of ethanol is stirred in a bath at 60 C. and 10 ml (10 mMol) of 1N sodium hydroxide solution are added. After 4 hours stirring at 60 C., 10 ml (10 mMol) of 1-N-hydrochloric acid are added in the warm and the mixture is left to cool to ambient temperature. After inoculation and standing overnight, the mixture is cooled for a further hour in ice, with stirring. The crystals are separated by suction filtering and washed twice with 5 ml of water. They are then dried at 75 C. up to a final temperature of 100 C./4 torr in a vacuum drying cupboard over phosphorus pentoxide. Yield: 3.13 g (87.7% of theory), Melting point: 130-131 C. (high-melting form) Calculated: C 71.64; H 8.02; N 6.19. Found: C 71.48; H 7.87; N 6.39. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









